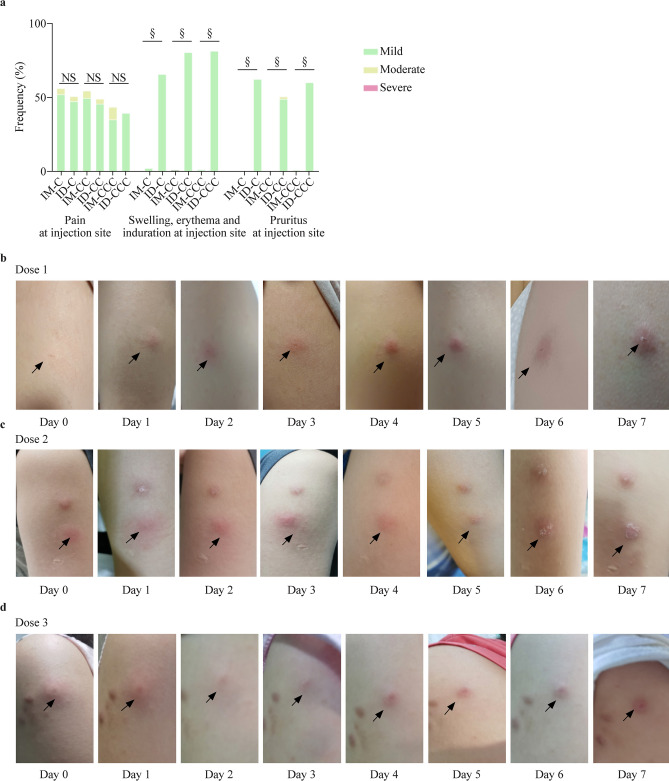
Fig. 5.
Local adverse reactions in the healthy safety population after doses 1, 2 and 3 of CoronaVac by intramuscular or intradermal administration. a Local adverse reactions 7 days after each dose of CoronaVac administered by intramuscular or intradermal injections were solicited from participants in the healthy safety population. Data are shown as percentages of the respective adverse reaction of any severity; b–d photos are representative of the typical injection site reactions manifested for 7 days after doses 1 (b), 2 (c) or 3 (d) of CoronaVac administered intradermally. IM-CC (n = 119) and IM-CCC (n = 94), post-dose 2 and post-dose 3 of vaccine administered intramuscularly, respectively; ID-CC (n = 59) and ID-CCC (n = 45), post-dose 2 and post-dose 3 of vaccine administered intradermally, respectively. ID intradermal, IM intramuscular, NS no significant difference. §P < 0.0001