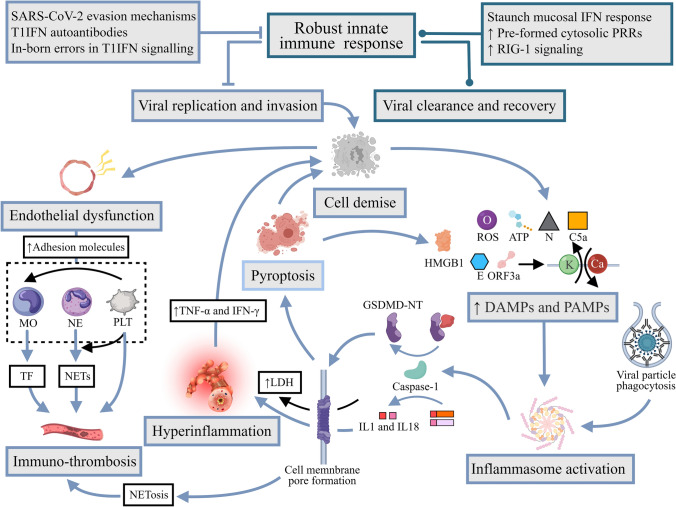
Fig. 3.
Hyperinflammation and immunothrombosis in severe COVID-19. Virus-related factors like the N protein and potassium efflux and calcium influx set off by envelope and ORF3a “viroporin” proteins, and host-related factors such as extracellular ATP, complement C5a, ROS, and phagocytosis of antibody-opsonized viral particles into otherwise ACE2− monocytes have all been proposed to trigger assembly of inflammasomes, most notably NLRP3, in myeloid-derived immune cells and pulmonary cells. Inflammasome activation recruits the caspase-1 canonically, which proteolytically potentiates pro-IL-1β and/or pro-IL-18 and concomitantly cleaves GSDMD into NT and CT fragments. GSDMD-NT oligomerization and translocation to plasma membrane create pores through which activated IL-1β and IL-18 can directly enter the extracellular space, mediating hyperinflammation, and simultaneously trigger pyroptosis, leading to LDH and HMGB1 release, among other pro-inflammatory DAMPs. Meanwhile, endothelial dysfunction in the hyperinflammatory milieu initiates immunothrombosis. Adhesion molecules are markers of activated endothelial cells over-expressed in COVID-19 that exhibit strong anchoring effects on monocytes and neutrophils, the former of which reciprocate with active TFs that directly institute the extrinsic coagulation pathway. Conversely, neutrophils may be prompted by direct SARS-CoV-2 entry, SAR-CoV-2-induced ROS generation, complement activation, and/or a self-sustaining loop of IL-8 production to undergo NETosis, defined by the release of large extracellular web-like structures termed NETs, which may also be triggered by the inflammasome/GSDMD pathway in COVID-19. NETs consist of decondensed chromatin embellished with histones and proteins that act as a scaffold for erythrocyte and platelet settling and fibrin deposition, while its constituents exert a range of pro-thrombotic effects with varying mechanisms. Furthermore, circulating platelets adopt a hyperactive state in the context of SARS-CoV-2 infection. Platelets recruited in response to NETs and other stimuli such as vWF on the activated endothelial cells in turn amplify NETosis via secretion of chemokines such as PF4, and may induce further expression of TF by monocytes and complementarily augment monocytic secretion of inflammatory cytokines. N protein nucleocapsid protein, ORF3a open reading frame 3a, ATP adenosine triphosphate, ROS reactive oxygen species, NLRP3 NOD-like receptor containing pyrin domain 3, IL-1β interleukin-1β, IL-18 interleukin-18, GSDMD gasdermin-D, NT N-terminal, CT C-terminal, LDH lactate dehydrogenase, HMGB1 high mobility group box 1, DAMPs damage-associated molecular patterns, TF transcription factor, IL-8 interleukin-8, NETs neutrophil extracellular traps, vWF von Willebrand Factor, PF4 platelet factor 4