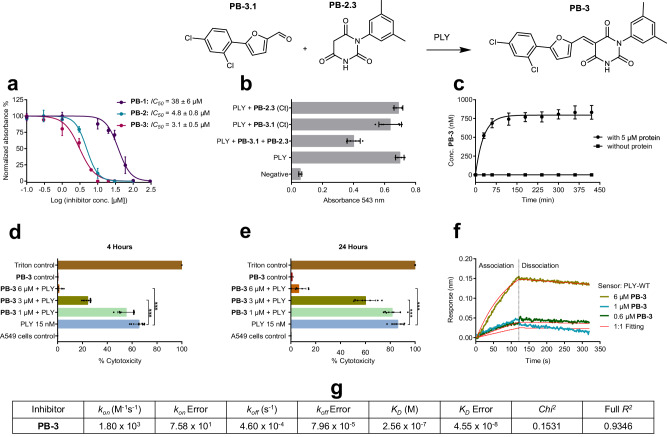
Fig. 2. The evaluation of activity and binding kinetics of PB-3.
a IC50 values of PB-1, PB-2, and PB-3 (distinct samples n = 3, 95% CI for IC50 and error bars represent ± S.D.) corresponding to the ability of PB-inhibitors to block the PLY-induced hemoglobin release in sheep erythrocytes. b PLY was inhibited by PB-3, formed via protein-templated fragment ligation in the hemolysis assay, while PB-3.1 and PB-2.3 alone were no inhibitors (distinct samples n = 3, error bars represent ± S.D.). c Quantification of PB-3 formed by protein-templated ligation using HPLC-QTOF-MS. Concentrations were fitted to the one-phase saturation equation c(t) = c0 + (cmax - c0)*(1-exp(-K*t)) used to obtain cmax (795 nM) and t1/2 (~20.5 min) (distinct samples n = 3, error bars represent ± S.D.). d, e PB-3 prevents human alveolar epithelial cells from PLY-associated impairment. Samples include controls (positive and negative), PLY alone, and PLY with PB-3 (1, 3 and 6 µM). Figures show LDH release (which is quantified as percent-cytotoxicity) after 4 h and 24 h. Mann Whitney test (two-tailed) is used for statistical evaluation (distinct samples n = 8, ***p = 0.0009 for d distinct samples n = 8 ***p = 0.0002 for (e), and error bars represent ± S.D.). f Binding kinetics of PB-3 and PLY complex measured in the BLI assay. PLY-C428A is used as reference protein and buffer is reserved as inhibitor reference. Binding is observed over 0.6, 1, and 6 µM of PB-3 (data shown are representing one of n = 3 distinct samples). The reference-subtracted data are presented in the graph, which display association and dissociation steps of wild-type PLY and PB-3. g The one-to-one kinetic fitting model was used to calculate the on- and off-rates (kon, koff) of binding and the binding affinity (KD) of PB-3 to PLY.