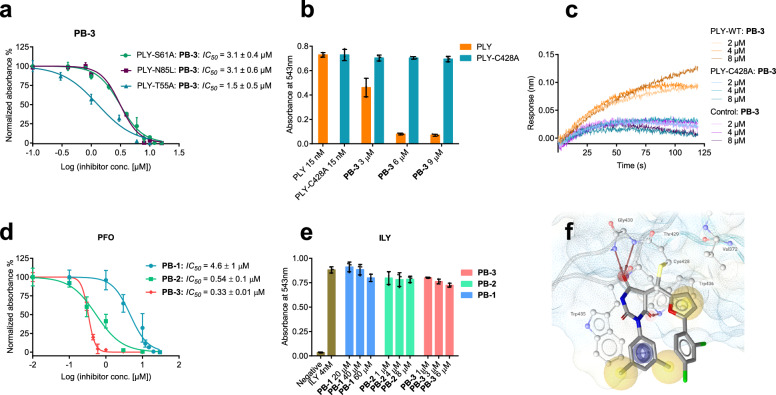
Fig. 4. Investigation of the biological activity and binding interactions of PB-inhibitors against PLY-mutants and evaluation of PB-inhibitors against alternate CDC.
a IC50 values of PB-3 against PLY-S61A, PLY-N85L, and PLY-T55A, which are mutants involving the potential binding site at the oligomerization interface (distinct samples n = 3, 95% CI for IC50 and error bars represent ± S.D.). b Comparison of PB-3 activity against wild-type PLY and PLY-C428A (a UDP-cysteine mutant version of PLY). PB-3 blocks only wild-type PLY and is unable to neutralize the mutant PLY-C428A at all three concentrations (distinct samples n = 3, error bars correspond to ± S.D.). c Bio-layer interferometry assay: wild-type PLY, PLY-C428A-immobilized, and control NiNTA biosensors exhibit association with PB-3 at three concentrations (2, 4, and 8 µM). Only PLY (wild-type) shows significant association with PB-3 in contrast to PLY-C428A (data presented are one of n = 3 distinct samples). d IC50 values of PB-1, 2, and 3 against perfringolysin (PFO, a toxin homologous to PLY) and PB-inhbitors show 10-fold increased potency toward PFO than PLY (distinct samples n = 3, 95% CI for IC50 and error bars represent ± S.D.). e Intermedilysin (ILY, a cysteine-free CDC toxin) is not blocked by PB-inhibitors (distinct samples n = 3 for each experiment, 95% CI for IC50 and error bars represent ± S.D.) f Covalent binding mode of PB-3 suggested docking, for details see text.