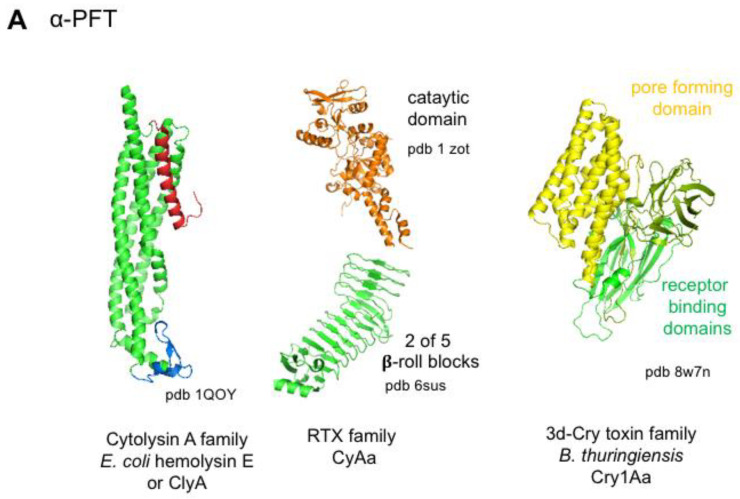
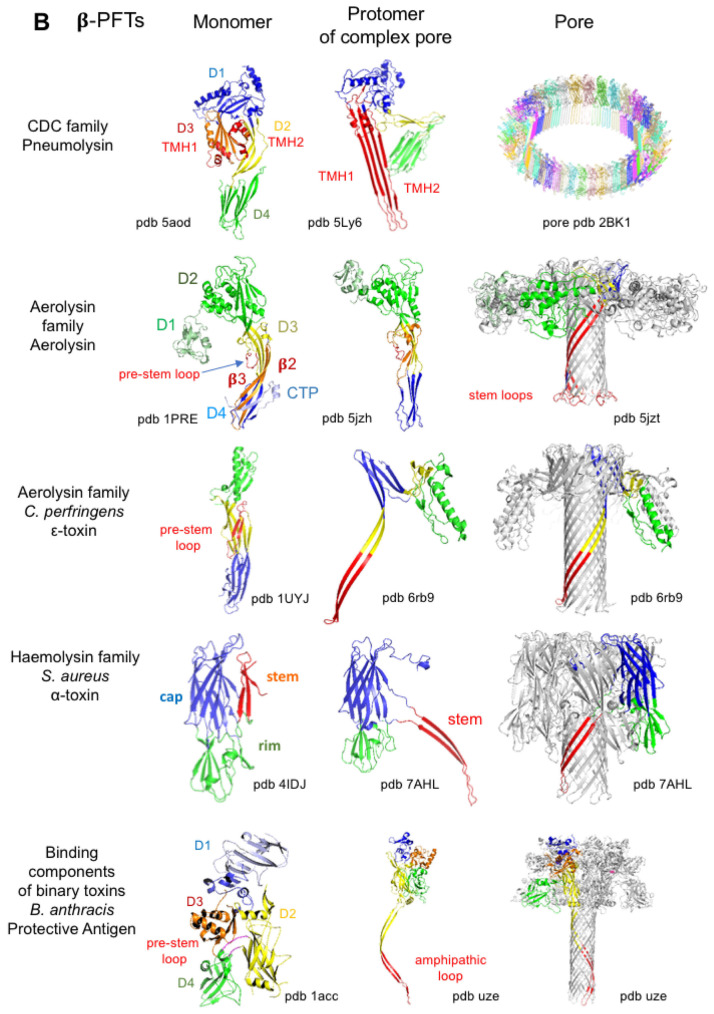
Figure 7.
Structures of representative α- and β-pore-forming toxins (PFTs) from the main PFT families. (A) Structures of soluble monomers of representative α-PFTs: E. coli hemolysin E or cytolysin A (ClyA), Bordetella pertussis adenylate cyclase (CyAa) (partial structure) from the RTX family, and B. thuringiensis Cry1Aa from the 3d-Cry toxins. (B) Structures of representative toxins from each β-PFT family showing soluble monomers, protomers from complex pores, and pores. Streptococcus peumoniae pneumolysin from the cholesterol-dependent cytolysins (CDCs). Each CDC monomer contains two α-helices, which change conformation into trans-membrane β-hairpin (TMH) upon oligomerization to form the β-barrel. Aerolysin contains an additional N-terminal domain (D1), which is missing in the other aerolysin family PFTs such as C. perfringens ε-toxin. S. aureus α-toxin and related toxins contain three domains with a more globular structure than the elongated aerolysin family PFTs. B. anthracis protective antigen (PA) is the binding component of B. anthracis edema and lethal toxins. PA structure with four domains is similar to that of PFO but PA monomer contains only one β-hairpin forming the β-barrel like β-PFTs other than CDCs. Figures were produced with the program MacPyMOL. Green, receptor binding domain; red, pore-forming domain; blue and yellow, oligomerization domains.