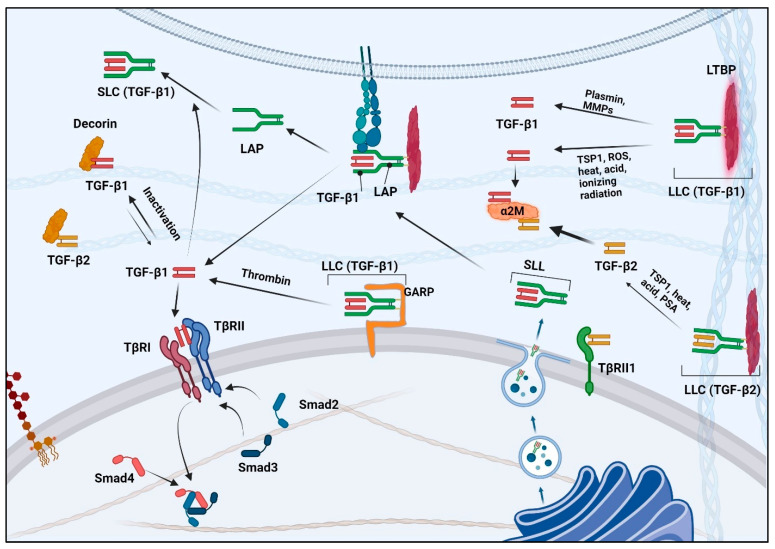
Figure 2.
The intricate processes of TGF-β activation within the intracellular and extracellular environments. Initially synthesized as homodimers with pro-peptides, mature TGF-βs are cleaved from latency-associated proteins (LAPs) by furin-like enzymes in the trans-Golgi. They are then secreted as small latent complexes (SLCs) bound to LAPs, often associating with latent TGF-β binding proteins (LTBPs) or glycoprotein A repetitions predominant (GARP) to form large latency complexes (LLCs) anchored to the extracellular matrix (ECM), or in the case of GARP, on the surface of specific cells. Activation of TGF-βs can occur via proteolytic cleavage or conformational changes induced by mechanical forces, integrins, reactive oxygen species (ROS), and other effectors. Notably, different isoforms of TGF-β are activated by distinct factors. Once activated, TGF-βs either bind to TGF-β receptors or are sequestered in an inactive form bound to extracellular matrix proteins such as decorin or the plasma protein alpha-2 macroglobulin (α2M), the latter of which has a 10-fold higher affinity for TGF-β2 than TGF-β1. Understanding the complexities of TGF-β activation offers insights into potential therapeutic interventions targeting aberrant TGF-β signaling.