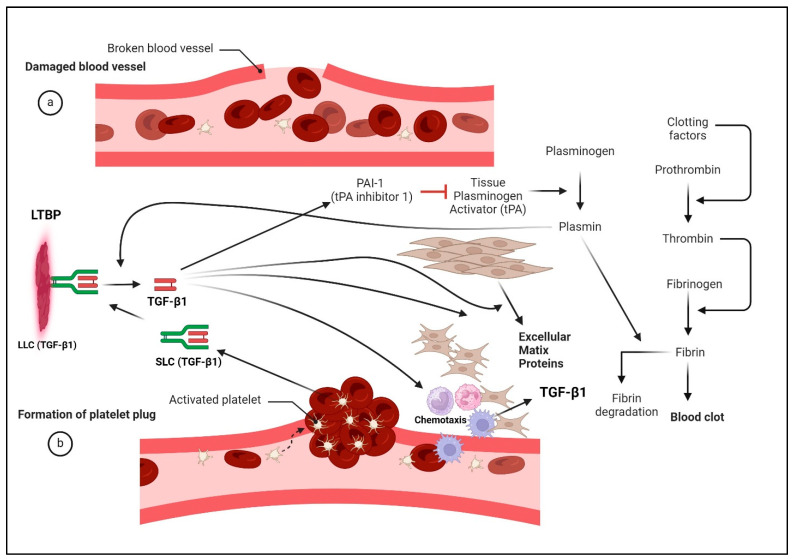
Figure 5.
The intricate involvement of TGF-β in wound healing, shedding light on its diverse roles and the complex interplay with various cellular processes. Platelets emerge as pivotal players, releasing TGF-β1 upon degranulation at the wound site, where it orchestrates a cascade of events. Tissue plasminogen activator (tPA) cleaves plasminogen into plasmin, which not only acts to limit the size of a blood clot by breaking down fibrin but also functions to activate TGF-β1 by cleaving it from its large latency complex (LLC). Here, activated TGF-β1 then acts as a chemoattractant for immune cells while simultaneously stimulating fibroblast proliferation and differentiation into myofibroblasts, which contribute to extracellular matrix deposition and wound repair. Despite its role in promoting wound repair, TGF-β1 induces immunotolerance, crucial for dampening autoimmunity triggered by tissue damage in normal tissue repair. TGF-β1 also drives the transcriptional induction of PAI-1 (tPA inhibitor-1), which functions to block the activation of plasmin, thereby limiting the extent of fibrin degradation. Tumors in which TGF-β1 is overexpressed/overactivated likely result in excess PAI-1 induction, which inhibits fibrin dissolution, thereby contributing to increased hypoxia and tissue damage.