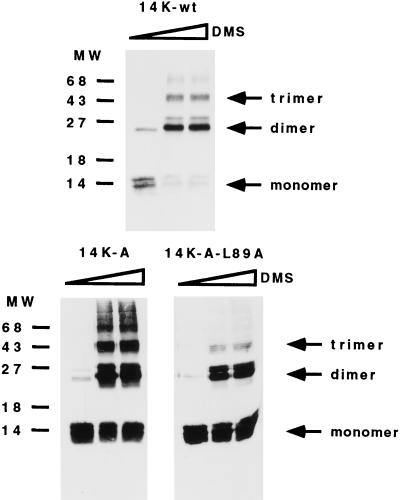
FIG. 5.
Oligomerization of the 14-kDa protein occurs independently of the cysteine residues. Cross-linking experiments were carried out with full-length E. coli-expressed 14-kDa wild-type protein and mutant forms 14K-A and 14K-A-L89A. Bacterial lysates obtained from 10-ml cultures of an E. coli expression system at 5 h postinduction were concentrated and resuspended in 100 μl of PBS. Different amounts of dimethyl suberimidate (DMS) (0, 2, and 5 μl) in dimethyl sulfoxide at a stock concentration of 50 mg/ml (freshly prepared) were added to the protein sample, and the mixtures were incubated on ice for 90 min. The reactions were quenched by addition of Tris-HCl (pH 8.0) to a final concentration of 20 mM, and the mixtures were then incubated at 4°C for 15 min. After the addition of sample buffer, proteins were analyzed on SDS–13% PAGE gels and profiles were revealed by Western blotting after reaction with MAbC3. Monomer, dimer, and trimer forms are indicated, and no differences were observed with increasing amounts of DMS. Molecular weight (MW) markers are on the left.