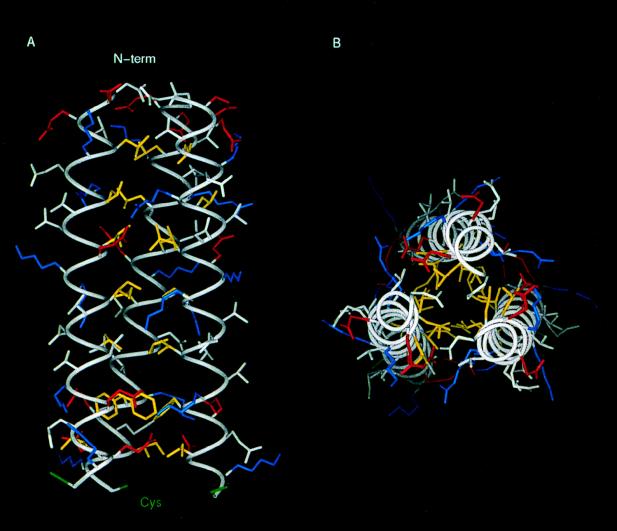
FIG. 6.
3-D model of the triple coiled-coiled region of the 14-kDa protein. Amino acid side chains are shown color coded according to their physical properties. Yellow, hydrophobics (Leu, Ile, Val, and Phe); Blue, positively charged residues (Arg and Lys); Red, negatively charged residues (Asp and Glu). Cys residues are in green and the rest of the residues are in white. (A) Longitudinal view. (B) Transversal view. The hydrophobic core can be seen in panel B, while in panel A the formation of ionic pairs between helices is shown.