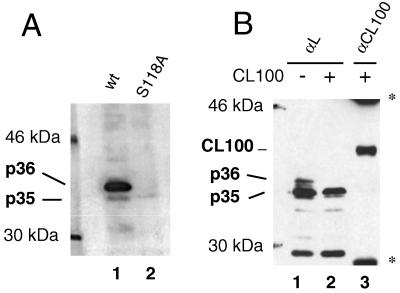
FIG. 4.
DHBV L is phosphorylated by an ERK-type MAP kinase. (A) In vitro kinase assay. Wild-type (wt) or S118A-DHBV particles were incubated with GST-tagged ERK2 and [γ32P]ATP in a reaction buffer which contained 0.5% NP-40 to disrupt the particles. L proteins were immunoprecipitated and separated by electrophoresis on an SDS–10% polyacrylamide gel, and radiolabeled proteins were detected by autoradiography. (B) Inhibition of phosphorylation at serine 118 by CL100, a phosphatase which specifically inactivates MAP kinases. COS7 cells were transfected with an L expression plasmid (pMT-DL) and a myc-tagged CL100 expression construct (pSG5-CL100myc) as indicated. Cells were lysed at day 2 posttransfection, and L proteins were immunoprecipitated and detected by Western blotting after separation on an SDS–10% polyacrylamide gel (lanes 1 and 2). To check for this presence, CL100myc protein was also immunoprecipitated from the lysate of cells cotransfected with L and CL100 and detected on the same Western blot (lane 3) (double stained with anti-pre-S and anti-myc). ∗, signals from mouse immunoglobulin G used for precipitating CL100myc.