Abstract
Thrombotic antiphospholipid syndrome (TAPS) is characterized by thrombosis and persistently positive tests for antiphospholipid antibodies or lupus anticoagulant (LAC). Triple-positive APS has the highest risk of recurrent thrombosis, but no studies have focused on recurrent thrombosis in patients with single-positive TAPS. We conducted a retrospective cohort study of patients with single-positive TAPS diagnosed at Lifespan Health System, Rhode Island, to determine the rates and risk factors for recurrent thrombosis. Between January 2001 and April 2022, 128 patients were assessed who had single-positive APS (LAC = 98, aCL = 21, aβ2GPI = 9) and who had been followed for a total of 1453.8 patient-years (median follow-up 3.04 years). The initial antithrombotic regimen was warfarin in 44%, a direct oral anticoagulant (DOAC) in 34%, enoxaparin in 2%, and no antithrombotic therapy or antiplatelet therapy only in 20%. Recurrent thrombosis occurred in 16 (12.5%) with a recurrent thrombosis rate of 3.08 per 100 patient-years. Systemic lupus erythematosus was the only variable signifantly associated with recurrent thrombosis in a model adjusted for age, sex, body mass index, and type of positive APS test. All 16 patients with recurrent thrombosis were initially treated with warfarin, and, at the time of recurrent thrombosis, 13 patients remained on warfarin and three were off anticoagulation. In conclusion, the recurrent thrombosis rate in single-positive APS is low, and not all patients with a single-positive test may need indefinite anticoagulation with warfarin. Larger prospective studies are required to confirm this finding and establish optimal anticoagulation regimens for low-risk TAPS.
INTRODUCTION
Antiphospholipid syndrome (APS) is an acquired thrombophilia characterized by thrombosis and/or pregnancy-related morbidity along with persistently positive antiphospholipid antibodies (anti-beta-2 glycoprotein inhibitor (aβ2GPI), anticardiolipin (aCL) antibodies) or lupus anticoagulant (LAC)1,2. Patients with APS with at least one thrombotic episode, known as thrombotic APS (TAPS), are at increased risk for recurrent thrombosis on stopping anticoagulation.3,4 Some studies also suggest a very high rate of thrombosis recurrence while these patients are receiving anticoagulation, but this was not seen in the warfarin (control) arm of recent clinical trials, which reported a 0–6.8% rate of recurrent thrombosis over a median follow up of 1–1.5 years.5–7 Thrombosis risk correlates with the number and types of positive antiphospholipid tests with patients with triple-positive APS (positive for LAC, aCL and aβ2GPI) at highest risk.8
Current guidelines, including those from the European League Against Rheumatism, the International Society of Thrombosis and Haemostasis (ISTH), and the British Society for Haematology, recommend long-term anticoagulation with a vitamin K antagonist for all patients with thrombotic APS9–12. These recommendations are based on a small set of key randomized clinical trials comparing the rates of recurrent thrombosis and bleeding events in patients on a direct oral anticoagulant (DOAC) to warfarin as the standard of care5–7,13. The trial of rivaroxaban in APS enrolled the highest risk group of triple-positive APS and failed to show non-inferiority of rivaroxaban compared with warfarin for secondary thrombosis prevention6. Subsequent studies that enrolled unselected patients with APS also could not establish the non-inferiority of rivaroxaban to VKAs for TAPS,5 and reported increased rates of thrombotic events, particularly arterial thrombotic events, in patients with APS treated with DOACs7.
None of the trials evaluating anticoagulation strategies in APS have focused exclusively on patients with a single-positive test to establish APS, or separately reported the outcomes of patients with only a single-positive serological test enrolled in other studies. Thus, patients with single-positive APS are often prescribed long-term warfarin therapy based on data derived from patients who may be selected for a higher risk of recurrent thrombosis. Long-term anticoagulation places patients at increased risk of major bleeding14, bleeding or thrombosis due to drug-drug interactions and imposes financial and monitoring burdens. While the benefit of indefinite anticoagulation in preventing recurrent thrombosis outweighs these risks for higher-risk patients with APS15, this may not be the case for those with APS established by a single-positive assay. To date, no studies have specifically evaluated rates and additional risk factors of recurrent thrombosis in patients with single-positive APS. The present study aims to identify risk factors and rates of recurrent thrombosis in single-positive APS.
METHODS
Study Design and Patients
We conducted a retrospective cohort study of consecutive adult patients (≥ 18 years of age) with single-positive thrombotic APS who received care with the Lifespan Health System in Rhode Island from Jan 1, 2001 to Apr 15, 2021. Potentially eligible subjects were identified via a query of the electronic medical record for patients with a history of Antiphospholipid syndrome (ICD-10-CM: D68.61), anticardiolipin syndrome (ICD-10-CM: D68.61), and lupus anticoagulant syndrome (ICD-10-CM: D68.62). A manual review of charts confirmed all diagnoses. We included only patients who met revised Sapporo criteria2 for thrombotic APS with positive testing for anticardiolipin IgG enzyme immunoassay (EIA) (>40 G phospholipid [GPL] units) and/or IgM (>40 M phospholipid units [MPL] or >99th percentile), beta-2-glycoprotein IgG and/or IgM enzyme-linked immunoassay (ELISA) (>40 Units [U] or >99th percentile), or lupus anticoagulant (per International Society on Thrombosis guidelines16) on at least two occasions at least 12 weeks apart, along with thrombotic events confirmed by review of diagnostic imaging. Our laboratory uses Hemosil AcuStar chemoiluminescent immunoassay kits for anti-beta-2-glycoprotein testing and lupus anticoagulant and QUANTA Lite enzyme-linked immunosorbent assay (ELISA) for anticardiolipin. Patients for whom laboratory diagnosis or thrombotic events were not confirmed were excluded from the analysis. Length of follow-up was considered to be the time of diagnosis of APS (time of second positive antiphospholipid antibody/LAC testing at least 12 weeks after initial testing) until death or last clinically relevant follow-up appointment, which occurred before Feb 2, 2022. The Rhode Island Hospital Institutional Review Board (IRB) approved the study.
Data Management and Study Outcomes
We extracted data from the electronic medical record, including patient demographics, details of APS diagnosis including circumstances and characteristics of thrombotic events, antithrombotic therapies, and the presence of comorbidities, including hypertension, hyperlipidemia, diabetes mellitus, morbid obesity (defined as body mass index >40 kg/m2), cancer, atrial fibrillation, peripheral vascular disease (PVD), cirrhosis, smoking, chronic kidney disease (CKD; defined as a glomerular filtration rate <60 mL/min per 1.73 m2 persisting over at least three months), oral contraceptive use, systemic lupus erythematosus (SLE), and other autoimmune diseases. Comorbidities (period prevalence at the end of follow-up) were determined by ICD-9/ICD-10 codes, documentation in health care provider notes or documented requirement of regularly scheduled medications for these disorders (along with documentation of the diagnosis).
The primary outcome was recurrent arterial or venous thrombosis, confirmed by a review of diagnostic imaging. In cases of recurrent thrombosis, we also recorded whether the recurrent event was provoked or unprovoked, the type of thrombosis (arterial or venous) and the antithrombotic regimen being taken at the time of thrombosis. For patients who developed recurrent thrombosis while on warfarin therapy, we evaluated whether the international normalized ratio (INR) was in the therapeutic range or subtherapeutic range in the 30 days before presentation with recurrent thrombosis. The therapeutic INR range was considered to be 2.0–3.0 as determined by prior randomized control trials in patients with TAPS17,18. Patients with an INR below the the therapeutic range on at least two or more occasions during this 30-day window were determined to be subtherapeutic.
Statistical Analysis
Data were summarized as counts and proportions for categorical data and medians and 25th–75th percentiles for continuous data. To minimize the effect of varying follow-up times, we calculated the incidence rate of recurrent thrombosis per 100 patient-years. First, patients with and without recurrent thrombosis were compared using Fisher’s exact tests for categorical data and Wilcoxon rank-sum tests for continuous data. Next, we evaluated risk factors for recurrent thrombosis in a Cox proportional hazards regression model. The date of APS diagnosis was set as time zero for the analysis, and covariates were selected based on significant association with recurrent thrombosis in univariate analysis or biologically plausible associations with recurrent thrombosis risks such as age, type of anticoagulant or type of positive antiphospholipid test (LAC, aCL or aβ2GPI). Freedom from recurrent thrombosis was graphed using Kaplan-Meier survival curves and compared between groups using the log-rank test. STATA version 17 (StataCorp) was used for all analyses. P < 0.05 was considered statistically significant.
RESULTS
Characteristics of the patient cohort
A total of 128 patients were evaluated who had APS established based on a single positive assay between Jan 2001 to Apr 2021. These patients were followed for a median of 3.04 years (interquartile range [IQR], 1.94–6.04). The total observation period for the cohort was 1453.8 patient years. The majority of patients were single-positive for LAC (n=98), with the remainder positive for aCL (n=21) and aβ2GPI (n=9). The mean age at APS diagnosis was 55 years (range: 19–92 years old), and 56% were female (Table 1). The median number of thrombotic events before APS diagnosis was 1 (range 0–4), and the first was venous thrombosis in most cases. The antithrombotic regimen started after the first thrombotic event was warfarin for 44.5% (N=57), a direct oral anticoagulant for 34.4% (N=44) and enoxaparin for 1.6% (N=2). There were also 25 patients (20.3%) who were either on no anticoagulation, or antiplatelet therapy alone, at the time of APS diagnosis. The most common reasons identified for the use of anti-platelet therapy alone was in patients with isolated positive anticardiolipin antibody and stroke or transient ischemic attack (TIA) or bleeding complications although data was limited by retrospective design. Characteristics of the patient cohort are summarized in table 1. (Table 1, Figure 1B).
Table 1:
Patient characteristics
| Characteristic | No Recurrent Clot | Recurrent Clot | p-value |
|---|---|---|---|
| N = 112 | N = 16 | ||
| Age, years (mean) | 55.5 (45, 64.5) | 55 (36, 69.5) | 0.942 |
| Female sex, n(%) | 61 (55%) | 10 (63%) | 0.600 |
| Type of APLA | |||
| LAC, n (%) | 88 (79%) | 10 (63%) | 0.205 |
| aCL, n (%) | 16 (14%) | 5 (31%) | 0.140 |
| aβ2GI, n (%) | 8 (7%) | 1 (6%) | 1.000 |
| Comorbidities | |||
| BMI 40.0 and above, n (%) | 19 (17%) | 4 (25%) | 0.486 |
| Estrogen containing oral contraceptives, n (%) | 5 (5%) | 0 (0%) | 1.000 |
| Systemic lupus erythematous, n (%) | 10 (9%) | 6 (38%) | 0.006 |
| Cancer, n (%) | 3 (3%) | 1 (6%) | 0.418 |
| Other thrombophilia, n (%) | 12 (11%) | 0 (0%) | 0.360 |
| Cigarette use, n (%) | 26 (23%) | 6 (38%) | 0.227 |
| Atrial fibrillation, n (%) | 5 (5%) | 2 (13%) | 0.212 |
| Peripheral vascular disease n (%) | 3 (3%) | 1 (6%) | 0.418 |
| Cirrhosis, n (%) | 0 (0%) | 0 (0%) | - |
| Hypertension, n (%) | 55 (49%) | 7 (44%) | 0.792 |
| Hyperlipidemia, n (%) | 40 (36%) | 4 (25%) | 0.575 |
| Chronic kidney disease, n (%) | 11 (10%) | 3 (19%) | 0.383 |
| Diabetes, n (%) | 11 (10%) | 1 (6%) | 1.000 |
| Characteristics of first thrombosis | |||
| Arterial Clot (initial), n (%) | 37 (33%) | 5 (31%) | 1.000 |
| Venous Clot (initial), n (%) | 70 (63%) | 11 (69%) | 0.784 |
| Initial antithrombotic regimen | |||
| Warfarin, n (%) | 41 (37%) | 16 (100%) | <0.001 |
| Enoxaparin, n (%) | 2 (2%) | 0 (0%) | 1.000 |
| Direct oral anticoagulants, n (%) | 44 (39%) | 0 (0%) | 0.001 |
| No anticoagulation or antiplatelet only, n (%) | 25 (22%) | 0 (0%) | 0.191 |
Figure 1. Recurrent thrombosis free survival by A) aPL profile (aCL, aβ2GPi, or LA) and B) Initial antithrombotic regimen.
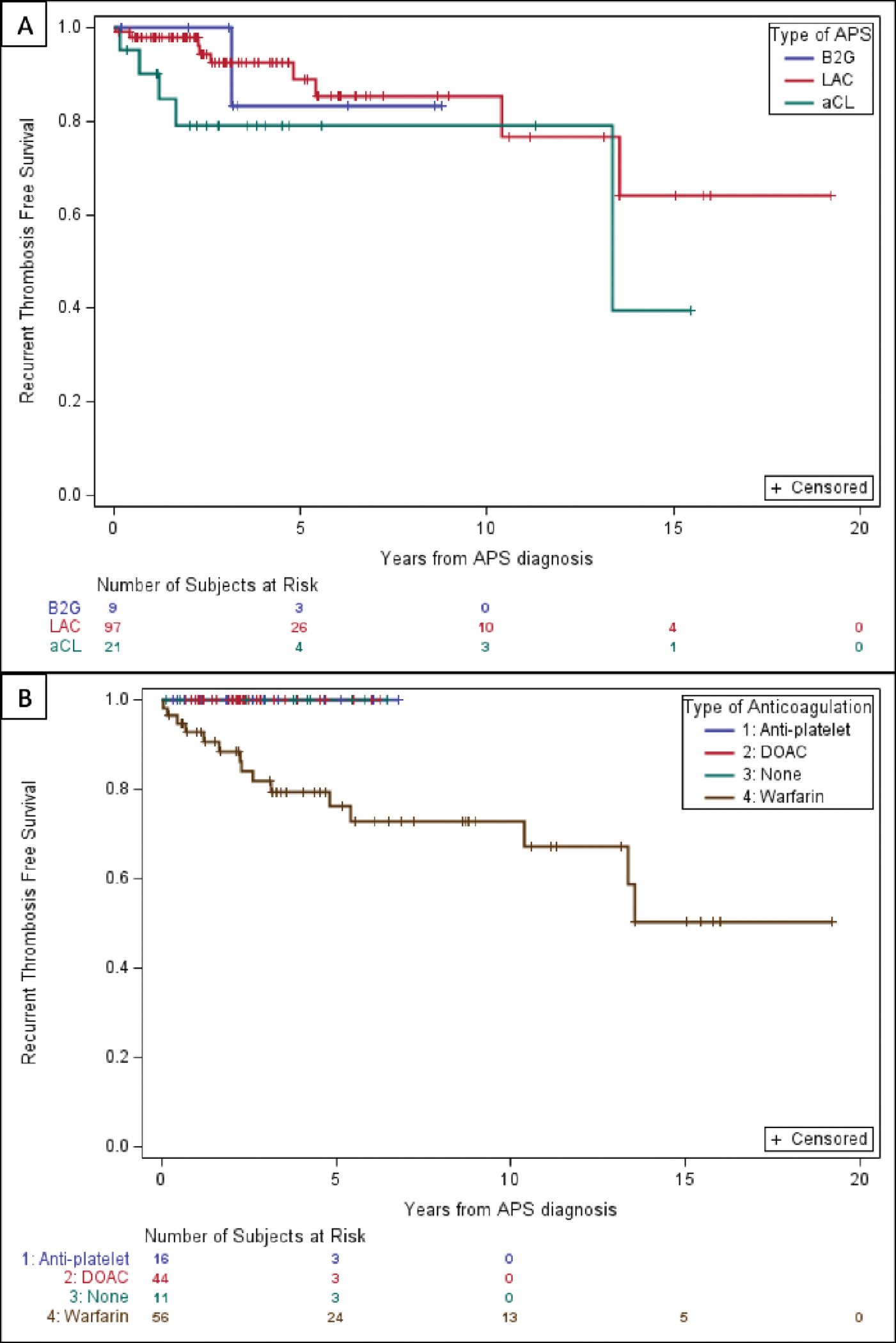
Predictors and characteristics of recurrent thrombosis
Recurrent thromboembolism occurred in 16 individuals (12.5%). The incidence of recurrent thrombosis was approximately 3 per 100 patient-years for the entire cohort. The recurrence rate was 2.52 per 100 patient-years (95% CI 0.96–4.08) among patients with single-positive LAC, 5.88 per 100 patient-years (95% CI 0.73–11.04) for single-positive aCL, and 2.58 per 100 patient years (95% CI −2.49–7.67) for single-positive aβ2GPI. Recurrent thrombosis-free survival by aPL profile is shown in figure 1A. All patients with recurrent thrombosis had been started on warfarin as their initial antithrombotic regimen. There was no difference in the distribution of type of antiphospholipid positivity in patients on DOACs versus warfarin. In our real world cohort, patients on warfarin were more likely to have systemic lupus erythematous (p=0.020) and more likely to have had an arterial clot at time of APS diagnosis than those initiated on DOACs (p<0.001) (Table 3) consistent with current guidelines on warfarin use in arterial thrombosis in APS5–7,13. Of these, 13 remained on warfarin at the time of recurrent thrombosis, and three patients had discontinued anticoagulation before the event (Figure 1B). Nine of the 13 patients on warfarin had INR data available for 30 days before the thrombotic event, of which 66.7% had at least two INRs below the accepted therapeutic range for patients with APS (2.0–3.0). Three patients with a recurrent clot had a clear provoking factor, which included recent surgery, hospitalization, or the presence of a central venous catheter.
Table 3:
Patient characteristics for DOAC versus Warfarin groups
| Characteristic | DOAC | Warfarin | p-value |
|---|---|---|---|
| N = 44 | N = 57 | ||
| Age, years (mean) | 58.5 (51, 68) | 57 (40, 65) | 0.260 |
| Female sex, n(%) | 18 (4%) | 39 (68%) | 0.008 |
| Type of APLA | |||
| LAC, n (%) | 36 (82%) | 38 (67%) | 0.110 |
| aCL, n (%) | 5 (11%) | 13 (23%) | 0.190 |
| aβ2GI, n (%) | 3 (7%) | 6 (11%) | 0.730 |
| Comorbidities | |||
| Estrogen containing oral contraceptives, n (%) | 2 (5%) | 2 (4%) | 1.000 |
| Systemic lupus erythematous, n (%) | 2 (5%) | 12 (21%) | 0.020 |
| Characteristics of first thrombosis | |||
| Arterial Clot (initial), n (%) | 3 (7%) | 21 (37%) | <0.001 |
| Venous Clot (initial), n (%) | 38 (86%) | 34 (59%) | 0.004 |
Systemic lupus erythematous [HR 6.30 (95% CI 1.42–27.84), p=0.015] was associated with recurrent thrombosis in a Cox regression model that also included type of antiphospholipid antibody test [LAC: HR 0.61 (95% CI 0.07–4.95), p=0.640; aCL : HR 0.34 (95% CI 0.32–28.54), p=0.336 with a β2GPI as the reference category, age [HR 0.99 (95% CI 0.96–1.03), p=0.678], sex [HR 0.63 (95% CI 0.17–2.27), p=0.476], and body mass index > 40 [HR 2.03 (95% CI 0.059–6.99), p=0.263].
DISCUSSION
In this study, we show for the first time that the rate of recurrent thrombosis in patients with single-positive APS is low regardless of antithrombotic strategy. All recurrent thrombosis occurred in patients initially treated with warfarin, with 13 of 16 patients continuing to receive warfarin at the time of their recurrent event; most of these events occurred in patients who had had a sub-therapeutic INR. The low rates of recurrent thrombosis suggest that selected patients who meet the criteria for APS (who have only a single-positive assay) may not require lifelong anticoagulation with warfarin, which presents the additional challenges of monitoring drug and food interactions.
The incidence rate of recurrent thrombosis in our single-positive APS cohort was lower than expected at approximately 3 per 100 patient-years. This rate of recurrent thrombosis is comparable to the 2 per 100 patient years rate of recurrent thrombosis in unselected patients with VTE treated with oral anticoagulation19. This rate is also much lower than the rates of recurrent thrombosis reported in APS cohorts at higher risk of recurrent thrombosis, which include patients with more than one positive antiphospholipid assay (double and triple-positive APS).20,21 We did not find a significant difference in the rates of recurrent thrombosis based on the type of antiphospholipid assay after adjusting for relevant variables including age, type of anticoagulant or type of positive antiphospholipid test.
In a prospective study of patients that discontinued anticoagulation after a first thrombotic event, Kearon et al. reported a relatively low recurrent thrombosis rate even in patients with single positive APS that discontinued anticoagulation (3.6 per 100 patient years for aCL only, 0.0 per 100 patient years for anti-beta2-glycoprotein-I only, and 7.4 per 100 patient years for LA only) that was comparable to the recurrent thrombosis rate in patients without antiphopholipid antibodies (4.5 per 100 patient years).21 These results, along with our findings, highlight that trials in higher risk APS may not be generalizable to single positive APS and this group deserves to be evaluated separately.
Of those patients who were initiated on warfarin at diagnosis, there was a higher recurrence rate (4.5 per 100 patient-years) versus other anticoagulation regimens (0.5 per 100 patient-years). There were no episodes of recurrent thrombosis in patients (86% with venous thrombotic APS) on DOACs. The finding that recurrent thrombotic events did not occur while on therapy with a DOAC is contrary to two recent retrospective studies focused on lower-risk (combined single-positive and double-positive) APS22,23. The first study in this patient cohort by Williams et al. showed higher rates of recurrent thrombosis in patients on a DOAC versus warfarin23. We later conducted a comporable study showing similar rates of recurrent thrombosis in patients with lower-risk APS on warfarin versus DOACs22. Earlier clinical trials of patients with high-risk APS showed inferiority of DOACs to warfarin in this population. The Trial of Rivaroxaban in Antiphospholipid Syndrome (TRAPS) that enrolled only patients with high-risk, triple-positive APS was stopped early due to increased rates of recurrent thrombosis in the rivaroxaban arm (12%) compared to no recurrent thrombosis in the warfarin group (0%).6 Ordi-Ros et al. randomized patients with thrombotic APS (single, double or triple positive) to rivaroxaban or warfarin reported a non-statistically significant near doubling in the rate of recurrent thrombosis in patients treated with rivaroxaban versus warfarin (11.6% versus 6.3%, respectively)5 and could not establish the non-inferiority of rivaroxaban. Of the 190-patient cohort, 60.5% were triple-positive. Finally, the Apixaban for Secondary Prevention of Thromboembolism Among Patients with Antiphospholipid Syndrome (APS-ASTRO) trial enrolled a more heterogeneous sample of APS (29.2% triple-positive) also revealed increased rates of recurrent stroke in patients treated with apixaban compared with those treated with warfarin7.
A potential explanation for the lower recurrence rate in our cohort of single-positive APS is that these trials enrolled higher risk APS and patients with single-positive APS are at lower risk for thrombosis at baseline. It is also likely that our patients on warfarin spent less time in the therapeutic range than patients in clinical trials; studies of patients treated with warfarin in the United States suggest that time in the therapeutic range ranges from 29–67%.24,25 Studies assessing non-adherence have showed differing rates with one finding a median rate of non-adherence of 14.4% (IQR 5.8–33.8)26 and another of 76.9% for all patients and 34.1% for patients with at least two warfarin prescriptions.27 DOACs, which prior research has shown to be suboptimal anticoagulation for most patients with APS, may be an acceptable alternative to warfarin for single positive APS with venous thrombosis, especially in those patients with whom INR control remains difficult. Given that our DOAC population almost exclusively included patients with venous thromboembolism at diagnosis, the lower recurrent thrombotic rates cannot be extrapolated to patients with single positive APS and arterial thrombosis.
SLE was associated with recurrent thrombosis in our cohort of single positive APS. This is consistent with previous reports that SLE is a risk factor for thrombosis in patients with an without APS.28–30 The higher proportion of patients with SLE on warfarin (21%) versus DOACs (5%) may help to explain the higher rates of recurrent thrombosis in the warfarin anticoagulation group and identify patients at higher risk of thrombosis in this otherwise lower-risk cohort. Additonally, some of the recurrent thrombotic events were provoked, highlighting the importance of continuing (or initiating) anticoagulation during high risk periods.
Our analysis is strengthened by the use of real-world evidence, including potential treatment interruptions that may play a role in the risk of recurrent thrombosis. However, it is limited by a retrospective study design and relatively small numbers. We cannot exclude selection bias where patients perceived by the treating clinician as ‘higher risk’ were preferentially prescribed warfarin. We could not ascertain if patients suffered recurrent thrombosis that was not captured by our methodology. We were also unable to determine time in the therapeutic range for all patients on warfarin since INRs were available only for the minority of patients who had INR monitoring by their hematologist or when INR was retrospectively documnented on presenting with recurrent thrombosis, which is a limitation attributable to the retrospective study design. Some of our patients with a positive LAC may have been “false positives” attributable to testing while receiving anticoagulation. We could only reliably assign patients to treatment groups based on the anticoagulation they received at the time of their APS diagnosis. Because of the small number of patients, we could not definitively evaluate the outcomes of patients who had discontinued antithrombotic therapy altogether. The data collection and analysis for this study were completed prior to the recent publication of the 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria.31 The use of the revised Sapporo criteria is similar to prior APS trials evaluating the use of DOACs allowing for straighforward comparison between studies. Future research should take into account these new criteria and the potential impact on the choice and duration of anticoagulation in this patient population. Finally, though fluctuations in aPL titers and even seroconversion from a positive to negative test has been described and may have particular relevance in the potentially lower risk group of single positive APS, serial aPL and LA testing was not systematically performed in this retrospective cohort so we were unable to examine the effect changes in aPL titer over time on thrombotic risk.32–34
In conclusion, single-positive APS appears to represent a lower-risk group that may not require indefinite Vitamin K antagonist therapy. Those with SLE are at higher risk of recurrent thrombosis than other patient groups. We did not find any patient initially treated with a DOAC, which predominantly represented patients with venous thrombotic APS, to have suffered recurrent thrombosis. Larger, prospective studies are needed to validate our findings and to identify optimal anticoagulation strategies for patients with single-positive APS.
Table 2:
Clinical characteristics and outcomes in patients with recurrent thrombosis
| Patient No. | Age/sex | aPL Type | SLE | OCP Use | Initial Clot | Initial AC | AC at time of Recurrent Clot | Recurrent Clot Type | Recurrent Clot Provoked | Subtherapeutic INR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37/F | LAC | + | − | DVT, PE | Warfarin | Warfarin | DVT | − | + |
| 2 | 59/F | LAC | − | − | DVT, PE | Warfarin | None | DVT | − | |
| 3 | 34/F | aCL | − | − | PE | Warfarin | Warfarin | PE | − | + |
| 4 | 80/F | aCL | − | − | CVA | Warfarin | Warfarin | MI | − | + |
| 5 | 55/F | LAC | − | − | DVT, PE | Warfarin | None | DVT, PE | − | |
| 6 | 30/F | LAC | + | − | CVA | Warfarin | Warfarin | Other arterial† | + | + |
| 7 | 74/M | aCL | − | NA | DVT, PE | Warfarin | Warfarin | DVT | − | N/A |
| 8 | 35/F | aβ2Gi | + | − | PE | Warfarin | Warfarin | Other venous | − | + |
| 9 | 49/M | LAC | − | NA | DVT | Warfarin | Warfarin | DVT | − | − |
| 10 | 74/F | LAC | + | − | DVT, PE | Warfarin | Warfarin | CVA | − | + |
| 11 | 30/F | aCL | + | − | DVT, PE | Warfarin | Warfarin | DVT | − | N/A |
| 12 | 55/M | LAC | − | NA | Other Arterial | Warfarin | Warfarin | Other arterial | − | − |
| 13 | 54/M | LAC | − | NA | Other Arterial | Warfarin | None | DVT† | + | |
| 14 | 65/M | aCL | − | NA | DVT | Warfarin | Warfarin | CVA | − | − |
| 15 | 64/M | LAC | − | NA | DVT | Warfarin | Warfarin | DVT† | + | N/A |
| 16 | 88/F | LAC | + | − | CVA | Warfarin | Warfarin | DVT | − | N/A |
= provoked, SLE = systemic lupus erythematous, OCP = oral contraceptive, AC = anticoagulation, LAC = lupus anticoagulant, aCL = anticardiolipin antibody, aβ2Gi = anti-beta-2 glycoprotein inhibitor, DVT = deep vein thrombosis, PE = pulmonary embolism, CVA = cerebrovascular accident, MI = myocardial infarction, NA = not applicable
Funding
SC is supported by National Heart Lung and Blood Institute grant K99HL15059 and an ASH Scholar award.
Footnotes
Conflict of Interest Disclosure
There were no conflicts of interest for Dr. Bakow and Lisa Yanek. Dr. Chaturvedi has received honoraria for consulting or advisory board participation from Alexion, Sanofi Genzyme, Sobi, Takeda, and UCB pharmaceuticals. Her institution has received research funding on her behalf from Takeda. She has also received honoraria/royalties from UpToDate.com and Dynamed.com.
In the last 36 months, Dr. Crowther has received Personal Funding or has sat on Advisory Boards for Astra Zeneca, Hemostasis Reference Laboratories, Syneos Health, and Eversana. He has prepared educational materials and/or presented talks for Bayer, Pfizer, and CSL Behring. He has participated in various medicolegal activities relating to thrombosis, anticoagulant drugs, or other aspects of hematological practice. He has also worked with multiple for-profit and not-for-profit entities such as Up To Date and medical communication companies. He holds the Leo Pharma Chair in Thromboembolism, endowed at McMaster University.
References
- 1.Chaturvedi S, McCrae KR. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. Nov 2017;31(6):406–417. doi: 10.1016/j.blre.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. Feb 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus. Feb 2011;20(2):206–18. doi: 10.1177/0961203310395803 [DOI] [PubMed] [Google Scholar]
- 4.Garcia D, Akl EA, Carr R, Kearon C. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood. Aug 1 2013;122(5):817–24. doi: 10.1182/blood-2013-04-496257 [DOI] [PubMed] [Google Scholar]
- 5.Ordi-Ros J, Saez-Comet L, Perez-Conesa M, et al. Rivaroxaban Versus Vitamin K Antagonist in Antiphospholipid Syndrome: A Randomized Noninferiority Trial. Ann Intern Med. Nov 19 2019;171(10):685–694. doi: 10.7326/M19-0291 [DOI] [PubMed] [Google Scholar]
- 6.Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. Sep 27 2018;132(13):1365–1371. doi: 10.1182/blood-2018-04-848333 [DOI] [PubMed] [Google Scholar]
- 7.Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. Mar 22 2022;6(6):1661–1670. doi: 10.1182/bloodadvances.2021005808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim W. Thrombotic risk in the antiphospholipid syndrome. Semin Thromb Hemost. Oct 2014;40(7):741–6. doi: 10.1055/s-0034-1390003 [DOI] [PubMed] [Google Scholar]
- 9.Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: Guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. Sep 2020;18(9):2126–2137. doi: 10.1111/jth.14935 [DOI] [PubMed] [Google Scholar]
- 10.PRAC Recommendations on Signals (2019).
- 11.Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. Oct 2019;78(10):1296–1304. doi: 10.1136/annrheumdis-2019-215213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arachchillage DRJ, Laffan M. What is the appropriate anticoagulation strategy for thrombotic antiphospholipid syndrome? Br J Haematol. Apr 2020;189(2):216–227. doi: 10.1111/bjh.16431 [DOI] [PubMed] [Google Scholar]
- 13.Cohen H, Hunt BJ, Efthymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. Sep 2016;3(9):e426–36. doi: 10.1016/S2352-3026(16)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. Sep 18 2014;124(12):1968–75. doi: 10.1182/blood-2014-04-571232 [DOI] [PubMed] [Google Scholar]
- 15.Crowther MA, Jones AE, Witt DM. Warfarin is the preferred therapy for patients with thrombotic APS: Back to the Future. J Am Coll Cardiol. Oct 22 2022;doi: 10.1016/j.jacc.2022.10.015 [DOI] [PubMed] [Google Scholar]
- 16.Devreese KMJ, de Groot PG, de Laat B, et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: Update of the guidelines for lupus anticoagulant detection and interpretation. J Thromb Haemost. Nov 2020;18(11):2828–2839. doi: 10.1111/jth.15047 [DOI] [PubMed] [Google Scholar]
- 17.Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost. May 2005;3(5):848–53. doi: 10.1111/j.1538-7836.2005.01340.x [DOI] [PubMed] [Google Scholar]
- 18.Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med. Sep 18 2003;349(12):1133–8. doi: 10.1056/NEJMoa035241 [DOI] [PubMed] [Google Scholar]
- 19.Schulman S How I treat recurrent venous thromboembolism in patients receiving anticoagulant therapy. Blood. Jun 22 2017;129(25):3285–3293. doi: 10.1182/blood-2017-03-742304 [DOI] [PubMed] [Google Scholar]
- 20.Dufrost V, Risse J, Reshetnyak T, et al. Increased risk of thrombosis in antiphospholipid syndrome patients treated with direct oral anticoagulants. Results from an international patient-level data meta-analysis. Autoimmun Rev. Oct 2018;17(10):1011–1021. doi: 10.1016/j.autrev.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 21.Kearon C, Parpia S, Spencer FA, et al. Antiphospholipid antibodies and recurrent thrombosis after a first unprovoked venous thromboembolism. Blood. May 10 2018;131(19):2151–2160. doi: 10.1182/blood-2017-09-805689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakow BR, Phung Q, Rabinovich D, Olszewski AJ, Reagan JL. Choice of anticoagulation in patients with low risk antiphospholipid syndrome. J Thromb Thrombolysis. Jul 2023;56(1):121–127. doi: 10.1007/s11239-023-02826-6 [DOI] [PubMed] [Google Scholar]
- 23.Williams B, Saseen JJ, Trujillo T, Palkimas S. Direct oral anticoagulants versus warfarin in patients with single or double antibody-positive antiphospholipid syndrome. J Thromb Thrombolysis. Jul 2022;54(1):67–73. doi: 10.1007/s11239-021-02587-0 [DOI] [PubMed] [Google Scholar]
- 24.Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Risk-adjusted percent time in therapeutic range as a quality indicator for outpatient oral anticoagulation: results of the Veterans Affairs Study to Improve Anticoagulation (VARIA). Circ Cardiovasc Qual Outcomes. Jan 1 2011;4(1):22–9. doi: 10.1161/CIRCOUTCOMES.110.957738 [DOI] [PubMed] [Google Scholar]
- 25.Singer DE, Hellkamp AS, Piccini JP, et al. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc. Feb 19 2013;2(1):e000067. doi: 10.1161/JAHA.112.000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Platt AB, Localio AR, Brensinger CM, et al. Can we predict daily adherence to warfarin?: Results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) Study. Chest. Apr 2010;137(4):883–9. doi: 10.1378/chest.09-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen SY, Wu N, Gulseth M, et al. One-year adherence to warfarin treatment for venous thromboembolism in high-risk patients and its association with long-term risk of recurrent events. J Manag Care Pharm. May 2013;19(4):291–301. doi: 10.18553/jmcp.2013.19.4.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazzan M, Vaccarino A, Marletto F. Systemic lupus erythematosus and thrombosis. Thromb J. 2015;13:16. doi: 10.1186/s12959-015-0043-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Zeinah G, Oromendia C, DeSancho MT. Thrombotic risk factors in patients with antiphospholipid syndrome: a single center experience. J Thromb Thrombolysis. Aug 2019;48(2):233–239. doi: 10.1007/s11239-019-01836-7 [DOI] [PubMed] [Google Scholar]
- 30.Danowski A, de Azevedo MN, de Souza Papi JA, Petri M. Determinants of risk for venous and arterial thrombosis in primary antiphospholipid syndrome and in antiphospholipid syndrome with systemic lupus erythematosus. J Rheumatol. Jun 2009;36(6):1195–9. doi: 10.3899/jrheum.081194 [DOI] [PubMed] [Google Scholar]
- 31.Barbhaiya M, Zuily S, Naden R, et al. The 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis Rheumatol. Oct 2023;75(10):1687–1702. doi: 10.1002/art.42624 [DOI] [PubMed] [Google Scholar]
- 32.Colling ME, Ay C, Kraemmer D, et al. Lupus anticoagulant test persistence over time and its associations with future thrombotic events. Blood Adv. May 24 2022;6(10):2957–2966. doi: 10.1182/bloodadvances.2021006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khawaja M, Magder L, Goldman D, Petri MA. Loss of antiphospholipid antibody positivity post-thrombosis in SLE. Lupus Sci Med. Oct 2020;7(1)doi: 10.1136/lupus-2020-000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Out HJ, Bruinse HW, Christiaens GC, et al. A prospective, controlled multicenter study on the obstetric risks of pregnant women with antiphospholipid antibodies. Am J Obstet Gynecol. Jul 1992;167(1):26–32. doi: 10.1016/s0002-9378(11)91619-6 [DOI] [PubMed] [Google Scholar]


