Abstract
Background
Cardioneuroablation (CNA) targeting ganglionated plexi has shown promise in treating vasovagal syncope. Only radiofrequency ablation has been used to achieve this goal thus far.
Objective
The purpose of this study was to investigate the utility of cryoballoon ablation (CBA) of the pulmonary veins (PVs) as a potential simplified approach to CNA.
Methods
We report our observations of autonomic modulation in a series of 17 patients undergoing CBA for atrial fibrillation and our early experience using CBA of the PVs in 3 patients with malignant vagal syncope. In 17 patients undergoing CBA of AF, sinus cycle length was recorded intraprocedurally after ablation of individual PVs.
Results
The most pronounced shortening of the sinus cycle length was observed after isolation of the right upper PV, which was ablated last. Reduced sinus node recovery time and atrioventricular (AV) nodal effective refractory period were observed after CBA. Resting heart rate was elevated by 6–7 bpm after CBA and persisted during 12-month follow-up. CBA of the PVs was performed in 3 patients with recurrent vagal syncope mediated by sinus arrest (n = 2) and AV block (n = 1). In all patients, isolation of the right upper PV resulted in marked shortening of sinus cycle length. During follow-up of 178 ± 43 days (134–219 days), CNA resulted in abolition of pauses, bradycardia-related symptoms, and syncope in all patients.
Conclusion
CBA of the PVs (particularly the right upper PV) may be a predictable anatomic CNA approach in patients with refractory vagal syncope due to sinus arrest and/or AV block and may warrant systematic investigation as a tool to perform CNA.
Keywords: Cardioneuroablation, Cryoballoon ablation, Autonomic modulation, Vasovagal syncope, Atrial fibrillation
Key Findings.
-
▪
Cryoballoon ablation of the right upper pulmonary vein may be a simplified, anatomic approach to cardioneuroablation targeting the superior paraseptal ganglionated plexus, which projects parasympathetic nerve fibers to the sinus node.
-
▪
Utilization of pulmonary vein cryoballoon ablation for cardioneuroablation may be particularly attractive for patients with vagal syncope who also have atrial fibrillation.
-
▪
The presented observational data are hypothesis-generating, and the utility of pulmonary vein cryoballoon ablation for cardioneuroablation will require careful prospective study to establish its safety and long-term efficacy.
Introduction
Regulation of cardiac physiological function is governed by the autonomic nervous system, with cardiac innervation converging at ganglionated plexi containing sympathetic and parasympathetic neurons. Excessive parasympathetic activity can lead to vasovagal syncope, mediated by either sinus pauses due to transient sinus arrest or sinoatrial exit block, or functional atrioventricular (AV) block, putting patients at risk for syncope or near-syncope, injury, and impaired quality of life frequently requiring pacemaker implantation.
Cardioneuroablation (CNA) is an emerging interventional therapy that targets the interface between the autonomic nervous system and the heart, particularly epicardial ganglionated plexi surrounding the caval veins and the atrial chambers.
Observational studies and limited controlled clinical trials of CNA have shown promising results in the treatment of refractory vasovagal syncope mediated by either transient sinus arrest or AV block. In these reports, ablation is performed exclusively using radiofrequency energy guided by anatomy and electroanatomic mapping. The long-term clinical outcomes of CNA currently are under investigation.
The concept of CNA emerged from observations made in patients undergoing ablative therapy of atrial fibrillation (AF). In recent years, we noted marked heart rate changes in individual patients undergoing cryoballoon ablation (CBA) of AF.
The objective of our study was to (1) demonstrate the effects of CBA of the individual pulmonary veins (PVs) on sinus cycle length, sinus node recovery time (SNRT), and AV nodal conduction properties in patients undergoing AF ablation; and (2) report our observations utilizing CBA of the PVs as a means of CNA in a small series of 3 patients with vagally mediated syncope.
Methods
Observational study of autonomic modulation in patients undergoing AF ablation
The AF study population consisted of 17 patients who underwent CBA for paroxysmal AF. All patients had symptomatic paroxysmal AF (self-terminating within 7 days) and had not responded to ≥1 antiarrhythmic drug or had contraindication for drug therapy. Antiarrhythmic drugs were discontinued for 5 half-lives before the procedure. The CBA procedure was performed with patients under general anesthesia without skeletal muscle relaxants. Transseptal catheterization and CBA were performed with standard technique using heparin anticoagulation and under guidance of intracardiac ultrasound. A deflectable decapolar catheter was placed in the coronary sinus. In all patients, a 28-mm CBA catheter (Arctic Front Advance, Medtronic Inc., Minneapolis, MN) was used to achieve PV isolation. An octapolar mapping catheter (Achieve, Medtronic Inc.) was used to guide PV isolation. The PVs were ablated in the following sequence: left upper, left lower, right lower, and right upper. The right phrenic nerve was constantly paced from the superior vena cava during freezing at the right-sided PVs. At the end of the procedure, PV isolation was confirmed by evidence of bidirectional conduction block and/or dissociated potentials.
Electrophysiological study was performed at baseline and after completion of CBA to assess sinus cycle length, SNRT (at drive cycle lengths of 600, 500, and 400 ms), AV nodal Wenckebach cycle length, and AV nodal and atrial effective refractory periods. Sinus cycle lengths were obtained at baseline and after completion of each PV isolation in all 17 patients. The average of 5 sinus cycle lengths was recorded. A vagal modulation response was defined as acute and sustained decrease in sinus cycle length >200 ms after PV ablation.
Antiarrhythmic drug therapy was resumed postprocedure. If no recurrence was noted at 2–3 months, antiarrhythmic therapy was discontinued. Patients underwent routine follow-up in the office with 12-lead surface electrocardiography at 1, 3, 6, and 12 months, and heart rates from electrocardiograms were obtained.
Observational study of autonomic modulation in patients undergoing CNA
Three patients with recurrent syncope and therapy-refractory vagotonic bradycardia underwent CNA using CBA exclusively. The primary objective of the CNA procedure for our patients was to ameliorate symptomatic bradycardia and pauses induced by excessive parasympathetic activity. Considering the young age of the patients and the desire to avoid permanent pacing, CNA was proposed and offered as an alternative treatment approach. The patients provided informed consent to undergo ablation as an experimental treatment for their neurocardiogenic syncope and all were offered a pacemaker implant as a guideline-directed alternative. All 3 patients opted to undergo ablative therapy in hopes of avoiding pacemaker implantation.
The procedures were performed with patients under conscious sedation or general anesthesia. Transseptal catheterization and CBA were performed using standard technique with heparin anticoagulation and under guidance of intracardiac ultrasound (as outlined for AF patients). The right phrenic nerve was constantly paced from the superior vena cava during CBA of the right-sided PVs. At the end of the procedure, PV isolation was confirmed by evidence of bidirectional conduction block and/or dissociated potentials. Endocardial voltage mapping before and after ablation was performed using a PentaRay (Biosense-Webster, Diamond Bar, CA) to document the lesion sets and assure complete PV isolation.
Statistical analysis
Continuous variables are given as mean ± SD. Continuous variables were compared using the Student t test and analysis of variance. Categorical variables were compared by χ2 analysis, Fisher exact test, or analysis of variance. P <.05 was considered significant.
Results
Observations in patients undergoing cryoballoon AF ablation
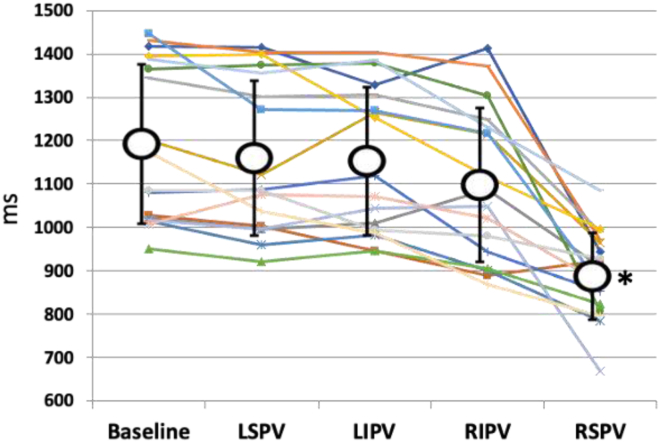
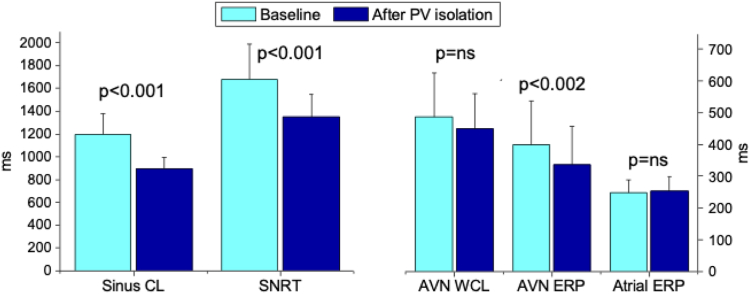
Seventeen patients underwent electrophysiological evaluation of sinus node and AV nodal function at baseline and after completion of PV isolation as well as serial recordings of sinus cycle length after isolation of the individual PVs. After CBA, there was a significant change in sinus cycle lengths only after right-sided PV isolation. Sinus cycle lengths changed from a baseline value of 1197 ± 183 ms to 1165 ± 178 ms, 1158 ± 170 ms, 1103 ± 177 ms, and 893 ± 100 ms after cryoablation of the the left superior PV, left inferior PV, right inferior PV, and right superior PV, respectively (P <.001 comparing right superior PV to baseline, left upper PV, left inferior PV, and right inferior PV) (Figure 1). After completion of CBA, SNRT shortened from a baseline value of 1682 ± 308 ms to 1351 ± 198 ms (P <.001) (Figure 2). Similarly, the AV nodal refractory period decreased from 399 ± 137 ms at baseline to 336 ± 122 ms after CBA (P <.002). AV nodal Wenckebach cycle length changed from 487 ± 138 ms at baseline to 449 ± 110 ms after CBA (P = .08) (Figure 2). There was no change in atrial effective refractory period after CBA. A decrease in sinus cycle length >200 ms suggesting modification of vagal innervation and impact on ganglionic plexi was observed in 12 of 17 patients (70%). The acute change in sinus cycle length after CBA correlated with changes in SNRT (r = 0.52; P <.03).
Figure 1.
Changes in sinus cycle length after sequential cryoballoon ablation of individual pulmonary veins. ∗P <.001 compared to baseline, LSVP, LIVP, and RIVP. LIVP = left inferior pulmonary vein; LSVP = left superior pulmonary vein; RIVP = right inferior pulmonary vein; RSVP = right superior pulmonary vein.
Figure 2.
Changes in electrophysiological parameters after cryoballoon atrial fibrillation ablation. AVN = atrioventricular node; CL = cycle length; ERP = effective refractory period; PV = isolation; SNRT = sinus node recovery time; WCL = Wenckebach cycle length.
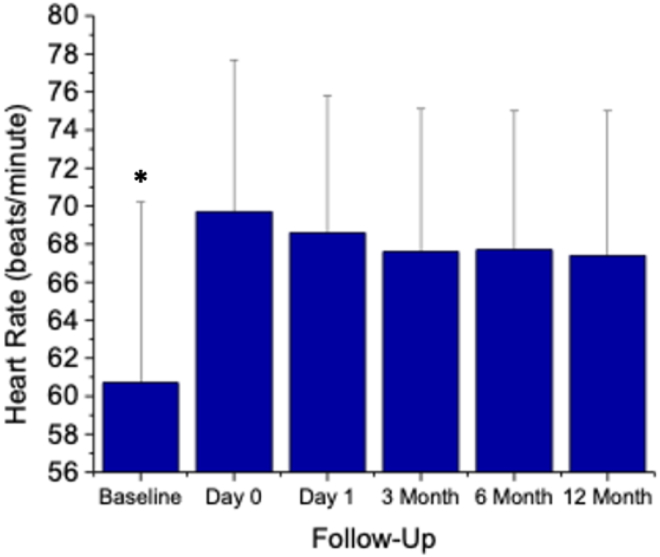
Resting heart rate increased after CBA from 61 ± 9 bpm before ablation to 70 ± 8 bpm after ablation (P <.001) and remained elevated on day 1 (69 ± 7 bpm) and at 3 months (68 ± 7 bpm), 6 months (68 ± 7 bpm), and 12 months (67 ± 7 bpm) of follow-up (Figure 3). At mean follow-up of 19 ± 11 months, 13 of 17 patients (76%) were free from AF recurrence, without antiarrhythmic therapy.
Figure 3.
Resting heart rate at baseline and during follow-up after cryoballoon atrial fibrillation ablation (n = 17). ∗P <.01 compared to days 0 and 1-, 3-, 6-, and 12-month follow-up.
Observations in patients undergoing cryoballoon CNA
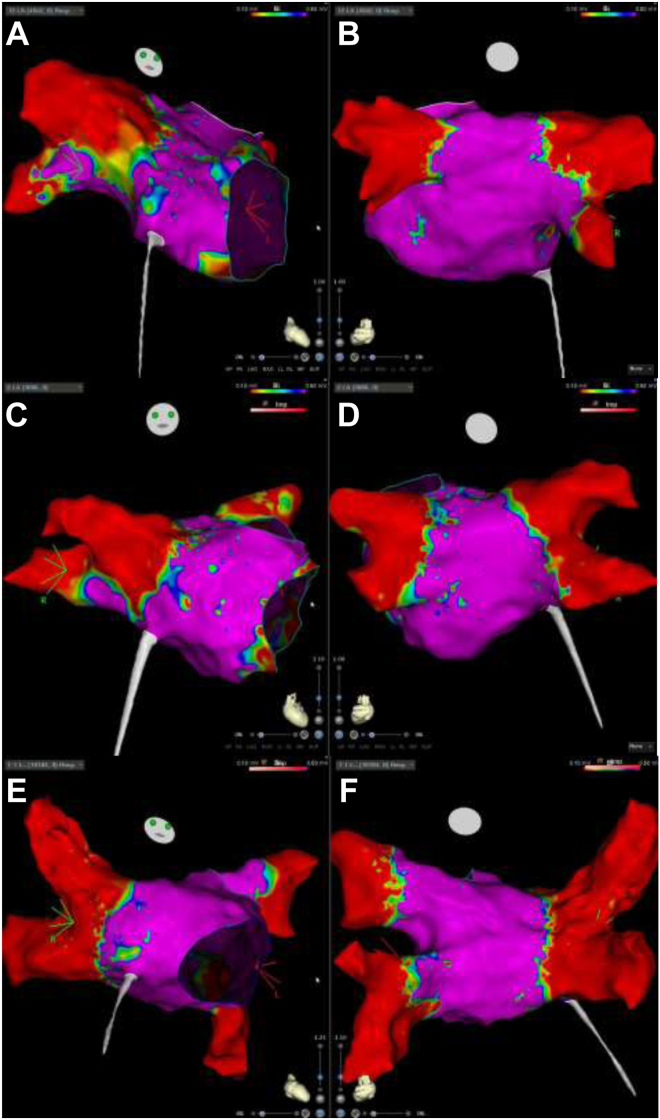
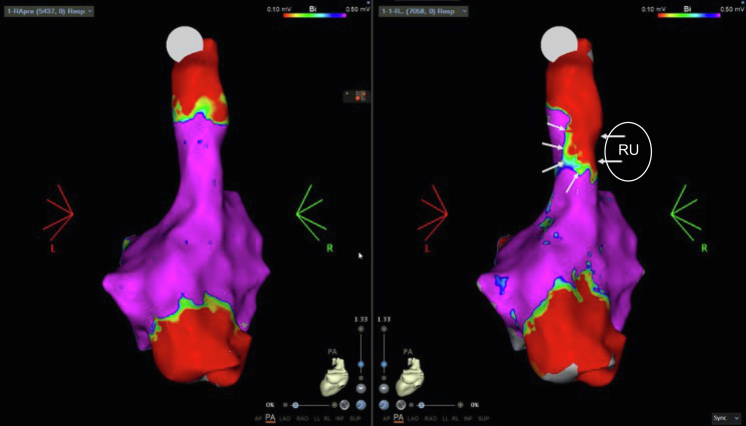
Clinical characteristics of the 3 patients who underwent cryoballoon CNA are summarized in Table 1. All patients had vagally induced paroxysmal bradycardia associated with syncope, which manifested as sinus arrest (patients 1 and 3) and AV block (patient 2). In patient 1, pauses occurred predominantly at night, related to obstructive sleep apnea. The observed pauses ranged from 6.3 to 28 seconds. All patients underwent CBA of the PVs using standard technique. In patients 1 and 2, only the right upper and left upper PVs were isolated. In patient 3, the right upper and right lower PVs were isolated. Endocardial voltage maps after ablation are shown in Figure 4. Right atrial mapping in patient 2 demonstrated the transmurality of cryoballoon lesions in the right upper PV, which is anatomically proximate to the superior paraseptal ganglionated plexus (Figure 5).
Table 1.
Clinical characteristics of patients undergoing cardioneuroablation
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age (y) | 35 | 45 | 64 |
| Sex | Male | Female | Male |
| Hypertension | No | Yes | No |
| Diabetes | No | No | No |
| Hyperlipidemia | No | No | No |
| Cardiomyopathy (history) | No | No | No |
| Atrial fibrillation (history) | No | No | No |
| Obesity | No | Yes | No |
| Thyroid disease (history) | No | No | No |
| Sleep apnea (obstructive or central) | Yes | Yes | No |
| Primary presentation | Recurrent syncope | Recurrent syncope | Recurrent syncope |
| Vagal | Yes | Yes | Yes |
| Sinus arrest | Yes | No | Yes |
| AV block | No | Complete AV block | No |
| Longest pause (s) | 11.2 | 6.3 | 28 |
| Ablation | Cryo only | Cryo only | Cryo only |
| Cryoablation | LU, RU | LU, RU | RU, RL |
| Follow-up (d) | 219 | 182 | 134 |
| Monitor pre/post | |||
| Monitor type | Holter/ILR | Holter/Holter | ILR/ILR |
| Days worn | 13/219 | 24/14 | 483/134 |
| Predominant rhythm | Sinus/sinus | Sinus/sinus | Sinus/sinus |
| HR minimum (bpm) | 32/71 | 22/67 | 30/70 |
| HR maximum (bpm) | 164/100 | 148/174 | 100/105 |
| HR mean (bpm) | 70/83 | 74/91 | 65/85 |
| Sinus pauses (no.) | 228/0 | 31/0 | 30/0 |
| AV block | No/no | Yes/no | No/no |
| AF/AFL burden (%) | 0/0 | 0/0 | 0/0 |
| Syncope (pre/post) | 3/0 | 2/0 | 3/0 |
AF = atrial fibrillation; AFL = atrial flutter; AV = atrioventricular; Cryo = cryoablation; HR = heart rate; ILR = implantable loop recorder; LU = left upper; RL = right lower; RU = right upper; SVC = superior vena cava.
Figure 4.
Left atrial endocardial voltage maps for patient 1 (A, B), patient 2 (C, D), and patient 3 (E, F) in the right anterior oblique (left) and posteroanterior (right) views.
Figure 5.
Right atrial endocardial voltage map (patient 3) before and after cryoballoon ablation in the left atrium (right upper [RU] pulmonary vein) demonstrating a transmural lesion (arrows) extending into the septal aspect of the superior vena cava–right atrial junction close to the location of the superior paraseptal ganglionated plexus.
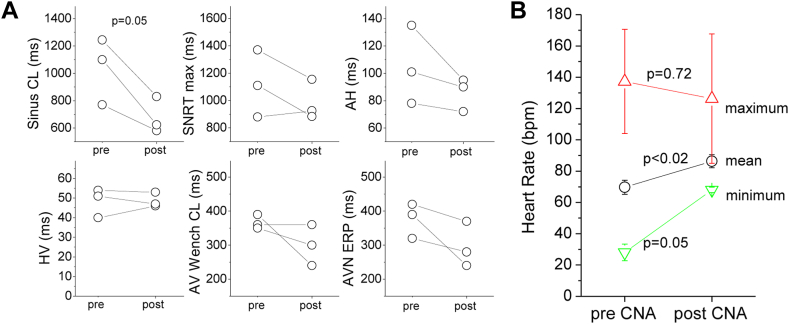
During the ablation procedure, sinus cycle length shortened from 1000 ± 180 ms to 687 ± 97 ms (P = .006) (Figure 6A). In 3 of 3 patients this occurred acutely after isolation of the right upper PV with CBA. We also observed a nonsignificant reduction of the maximum SNRT (from 1121 ± 264 ms to 983 ± 112 ms; P = .18), AH interval (from 98 ± 22 to 84 ±9 ms; P = .09), AV nodal Wenckebach cycle length (from 392 ± 43 to 332 ± 61 ms; P = .08), and AV nodal effective refractory period (from 337 ± 89 to 277 ± 66 ms; P = .16) (Figure 6A).
Figure 6.
Findings at the time of electrophysiological study and during long-term monitoring before and after ablation in patients undergoing cryoballoon cardioneuroablation (CNA). A: Observations during electrophysiological study pre- and post cryoballoon CNA. B: Ambulatory monitoring shows a marked increase in mean and minimum heart rate and a mild decrease in maximum heart rate as a result of the CNA procedure, demonstrating overall reduced heart rate variability. AV = atrioventricular; AVN = atrioventricular node; CL = cycle length; ERP = effective refractory period; SNRT = sinus node recovery time; Wench = Wenckebach.
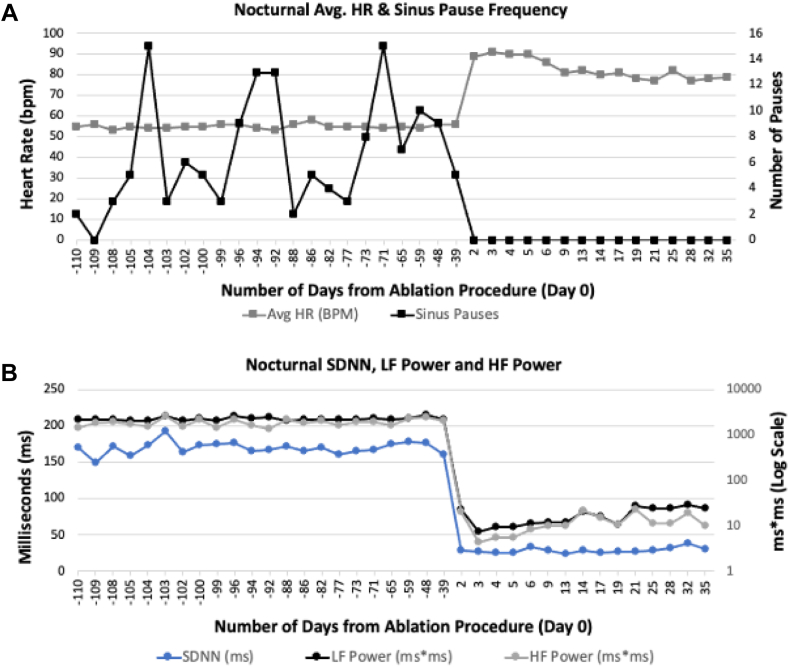
Ambulatory monitoring performed before and after the procedure demonstrated an increase in mean heart rate from 69 ± 5 bpm to 86 ± 4 bpm (P <.02), accompanied by an increase in minimal heart rate from 28 ± 5 bpm to 69 ± 2 bpm (P = .002) and a nonsignificant decrease in maximal heart rate from 137 ± 33 bpm to 126 ± 41 bpm (P = .7) indicative of decreased heart rate variability (Figure 6B). Ambulatory monitoring and clinical follow-up of 178 ± 43 days (134–219 days) revealed an absence of pauses and syncope in all patients. Patient 1, who suffered from obstructive sleep apnea, performed nightly monitoring using a CONTEC 3-lead Holter monitor (Contec Medical SystemsCo., Ltd., Quinhuangdao, China) before and after ablation (Figure 7). Nocturnal monitoring showed a marked increase in heart rate after ablation, abolition of all pauses (Figure 7A), and a marked reduction in heart rate variability (Figure 7B). In summary, the 3 patients were satisfied with their procedural result.
Figure 7.
A: Nocturnal average heart rate (HR) and frequency of sinus pauses during sleep in patient 1, monitored using a CONTEC 3-lead Holter monitor, before (–110 to 0 days) and after (0 to 57 days) cardioneuroablation. The day of ablation is designated as day 0. Sinus pauses are classified as >2.5 seconds. Each HR datapoint represents the collective average derived from hourly average values. The prominent reduction in sinus pause frequency and concomitant increase in heart rate postprocedure is evident. B: Alterations in nocturnal heart rate variability parameters before and after cardioneuroablation in patient 1: SDNN (ms), LF power (ms∗ms in logarithmic scale), and HF power (ms∗ms in log scale) before (–10 to 0 days) and after (0 to 57 days) ablation. A consistent decrease among all 3 heart rate variability parameters is observed postprocedure. HF = high-frequency band; LF = low-frequency band; SDNN = standard deviation of NN intervals.
Discussion
In this study, we present evidence of autonomic modulation in a cohort of patients undergoing CBA of AF as well as our experience with using CBA for CNA in 3 patients with vagal syncope. In patients undergoing CBA of AF, we observed the most significant increase in heart rate after CBA of the right upper PV associated with marked shortening of the SNRT and AV nodal effective refractory period. Elevation of heart rate after CBA of AF is sustained at follow-up of 12 months. In our 3 patients CBA-CNA of the PVs resulted in complete abolition of vagal syncope, symptomatic bradycardia, and pauses due to sinus arrest or AV block, as well as a sustained increase in heart rate at follow-up >5 months. Notably, CBA of the right upper PV resulted in a transmural scar that extended into the septal aspect of the superior vena cava–right atrial junction close to the location of the superior paraseptal ganglionated plexus as demonstrated by right atrial voltage mapping (patient 3; Figure 5). Based on the data presented here, we believe that CBA of the PVs (CBA of the PVs (particularly the right upper PV) may warrant systematic investigation particularly the right upper PV) may warrant systematic investigation as a tool to accomplish CNA in patients with refractory vagal syncope due to sinus arrest and/or AV block. CBA may be particularly attractive for patients with vagal syncope and coexistent AF.
Since the initial report by the Pachon et al1 in 2004, the technique of radiofrequency CNA has been refined. However, the current evidence for the technique in patients with severe drug-refractory vasovagal syncope is limited to small observational studies and 1 randomized controlled trial, but all consistently reported a significant reduction in syncope after CNA.2, 3, 4, 5, 6, 7, 8, 9, 10, 11 None has described using CBA as an adjunct modality for ablation.
A recent review by Brignole et al12 emphasized that the main target for CNA is the superior paraseptal ganglionated plexus (located at the junction between the interatrial septum and the superior vena cava), which is the final common pathway of the right vagus innervating the sinoatrial node. The inferior paraseptal ganglionated plexus (located near the proximal coronary sinus ostium and the posteroseptal junction between the right atrium and inferior vena cava adjacent to posteroinferior left atrium at the so-called pyramid space) is the final common pathway of the left vagus innervating the AV node. It is unclear whether this can be accomplished by right atrial ablation only or will require left atrial or biatrial ablation.12
Furthermore, which ablation modality should be used is not established. Our observations during CBA of AF are in accordance with observations made by Garabelli et al.13 The surface area of all 4 of the major atrial ganglionated plexi (determined by response to high-frequency stimulation) was substantially reduced by CBA. The superior left and anterior right ganglionated plexus had the largest whereas the inferior left ganglionated plexus had the least percentage of reduction after CBA. In the present study, we demonstrated that sinus cycle length shortening was most pronounced after CBA of the right upper PV. Furthermore, we demonstrated that the effect is sustained over 12 months.
This is the first report of intentional CNA in patients with vasovagal syncope achieved by CBA of the PVs. Our observations in patients with AF as well as patients with vasovagal syncope suggest that CBA of the right-sided PVs, particularly the right upper PV, is most critical to achieve modulation of vagal innervation. Utilization of CBA as a means of CNA may be particularly attractive in patients with vagal syncope who also have AF. This scenario may be more common than previously thought, considering that vagotonia also promotes AF. In fact, 2 of our 3 patients with vagal syncope reported had sustained AF during the procedure initiated by catheter manipulation in the absence of a clinical history of AF.
Identification of the location of ganglionated plexi by mapping of fragmented electrograms often is complicated and disappointing, with high-frequency stimulation frequently resulting in induction of AF. Thus, a primarily anatomic approach to CNA may simplify this procedure dramatically. The cryoballoon is a device with an established safety profile that may create a deep lesion that involves the epicardial tissue adjacent to the superior vena cava and the aorta containing critical parasympathetic fibers. Zarzoso et al14 demonstrated right upper PV ganglia projecting postganglionic nerves to the sinoatrial node by tyrosine hydroxylase and choline-acetyltransferase immunofluorescence labeling. Therefore, CBA of the right upper PV may be a good initial approach to achieve modification of vagal inputs into the sinoatrial node. CBA of the right-sided PVs (or right upper PV alone) may be sufficient to achieve success in CNA, but what endpoints should be observed to predict success and whether additional ablation targets are required are important questions to answer in carefully designed prospective studies. Furthermore, the long-term consequences of radical vagal denervation have not been sufficiently investigated.15
Study limitations
The data presented here are retrospective and observational in nature. Although the approach to CNA ablation included CBA in all patients, the ablation approach was variable. Therefore, the presented data should be viewed as hypothesis-generating. No conclusive evidence is presented that would allow us to recommend a specific ablation strategy using CBA only. Well-designed prospective randomized trials are needed to investigate this further. Previous investigators used atropine challenge and extracardiac vagal stimulation to help define ablation endpoints and determine procedural success. This was not done in the present study and should be part of a prospective evaluation of the potential role of CBA for CNA.
Conclusion
Our observations in a cohort of patients with AF and in a small series of patients with cardioinhibitory vagal syncope suggest that CBA of the PVs, particularly the right upper PV, can result in vagal modulation resulting in a sustained increase in heart rate and abolition of vagotonic pauses. Prospective clinical trials are needed to establish whether and how CBA can be used during CNA.
Acknowledgment
The authors would like to thank Connor Dennewitz for his invaluable help with formatting the figures included in this study.
Funding Sources
The authors have no funding sources to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
The patients provided informed consent to undergo ablation as an experimental treatment for their neurocardiogenic syncope, and all were offered a pacemaker implant as a guideline-directed alternative.
Ethics Statement
This study was approved by the Institutional Review Board at both involved institutions and conducted according to the guidelines outlined in the Declaration of Helsinki.
References
- 1.Pachon J.C., Pachon E.I., Pachon J.C., et al. “Cardioneuroablation”: new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2004:1–13. doi: 10.1016/j.eupc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Pachon P.C., Pachon E.I., Aksu T., et al. Cardioneuroablation: where are we at? Heart Rhythm O2. 2023;4:401–413. doi: 10.1016/j.hroo.2023.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pachon J.C., Pachon E.I., Cunha Pachon M.Z., Lobo T.J., Pachon J.C., Santillana T.G. Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: cardioneuroablation long-term results. Europace. 2011;13:1231–1242. doi: 10.1093/europace/eur163. [DOI] [PubMed] [Google Scholar]
- 4.Pachon M.E., Pachon-Mateos J.C., Higuti C., et al. Relation of fractionated atrial potentials with the vagal innervation evaluated by extracardiac vagal stimulation during cardioneuroablation. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007900. [DOI] [PubMed] [Google Scholar]
- 5.Piotrowski R., Baran J., Sikorska A., Krynski T., Kulakowski P. Cardioneuroablation for reflex syncope: efficacy and effects on autonomic cardiac regulation—a prospective randomized trial. JACC Clin Electrophysiol. 2023;9:85–95. doi: 10.1016/j.jacep.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Aksu T., Guler T.E., Bozyel S., Yalin K., Gopinathannair R. Usefulness of post-procedural heart rate response to predict syncope recurrence or positive head up tilt table testing after cardioneuroablation. Europace. 2020;22:1320–1327. doi: 10.1093/europace/euaa230. [DOI] [PubMed] [Google Scholar]
- 7.Huang X., Chen Y., Huang Y., et al. Comparative effects of intensive ganglionated plexus ablation in treating paroxysmal atrial fibrillation and vasovagal syncope. Clin Cardiol. 2020;43:1326–1333. doi: 10.1002/clc.23446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L., Zhao Y., Duan Y., et al. Clinical efficacy of catheter ablation in the treatment of vasovagal syncope. J Clin Med. 2022;11:5371. doi: 10.3390/jcm11185371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calo L., Rebecchi M., Sette A., et al. Catheter ablation of right atrial ganglionated plexi to treat cardioinhibitory neurocardiogenic syncope: a long-term follow-up prospective study. J Interv Card Electrophysiol. 2021;61:499–510. doi: 10.1007/s10840-020-00840-9. [DOI] [PubMed] [Google Scholar]
- 10.Debruyne P., Rossenbacker T., Janssens L., et al. Durable physiological changes and decreased syncope burden 12 months after unifocal right-sided ablation under computed tomographic guidance in patients with neurally mediated syncope or functional sinus node dysfunction. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.120.009747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candemir B., Baskovski E., Beton O., et al. Procedural characteristics, safety, and follow-up of modified right-sided approach for cardioneuroablation. Anatol J Cardiol. 2022;26:629–636. doi: 10.5152/AnatolJCardiol.2022.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brignole M., Aksu T., Calò L., et al. Clinical controversy: methodology and indications of cardioneuroablation for reflex syncope. Europace. 2023;25:1–10. doi: 10.1093/europace/euad033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garabelli P., Stavrakis S., Kenney J.F.A., Po S.S. Effect of 28-mm cryoballoon ablation on major atrial ganglionated plexi. JACC Clin Electrophysiol. 2018;4:831–838. doi: 10.1016/j.jacep.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Zarzoso M., Rysevaite K., Milstein M.L., et al. Nerves projecting from the intrinsic cardiac ganglia of the pulmonary veins modulate sinoatrial node pacemaker function. Cardiovasc Res. 2013;99:566–575. doi: 10.1093/cvr/cvt081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung W.H., Masuyama K., Challita R., et al. Ischemia-induced ventricular proarrhythmia and cardiovascular autonomic dysreflexia after cardioneuroablation. Heart Rhythm. 2023;20:1534–1545. doi: 10.1016/j.hrthm.2023.08.001. [DOI] [PubMed] [Google Scholar]