FIGURE 2.
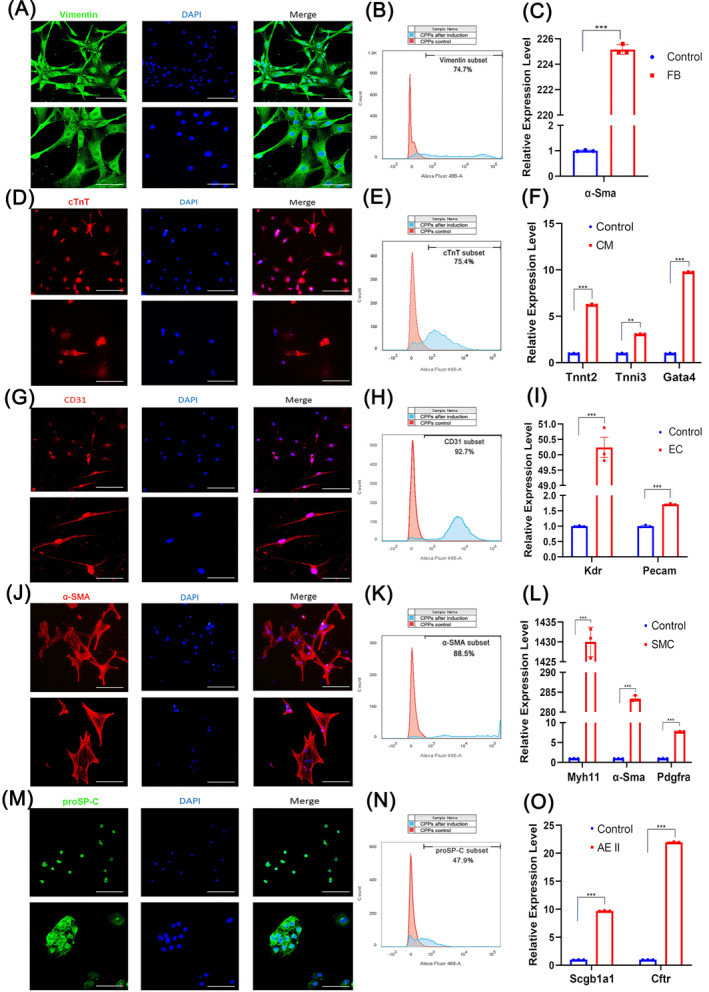
Identification of cardiopulmonary progenitors (CPPs') potential for multiple differentiation. (A–C) Immunofluorescence, flow cytometry and quantitative reverse transcription‐polymerase chain reaction (RT‐qPCR identification of markers for fibroblasts (Vimentin for immunofluorescence and flow cytometry, and α‐SMA for RT‐qPCR) in CPPs inducted into fibroblasts. (D–F) Immunofluorescence, flow cytometry and RT‐qPCR identification of markers for cardiomyocytes (cTnt for immunofluorescence and flow cytometry, and Tnnt2, Tnnl3, and Gata4 for RT‐qPCR) in CPPs induced into cardiomyocytes. (G–I) Immunofluorescence, flow cytometry and RT‐qPCR identification of markers for endothelial cells (CD31 for immunofluorescence and flow cytometry, and Kdr and Pecam for RT‐qPCR) in CPPs induced into endothelial cells. (J–L) Immunofluorescence, flow cytometry and RT‐qPCR identification of markers for smooth muscle cells (α‐SMA for immunofluorescence and flow cytometry, and Myh11, α‐Sma, and Pdgfra for RT‐qPCR) in CPPs induced to smooth muscle cells. (M–O) Immunofluorescence, flow cytometry and RT‐qPCR identification of markers for type II alveolar epithelial cells (proSP‐C for immunofluorescence and flow cytometry, and Scgb1a1 and Cftr for RT‐qPCR) in CPPs induced to type II alveolar epithelial cells. For (A, D, G, J, and M), upper images: scale bar, 200 μm; lower images: scale bar, 100 μm. For (G, F, I, L, and O), data are shown as the mean ± SEM.; n = 3 biological replicates per group; **p < 0.01, ***p < 0.001 (t‐test). CM, cardiomyocyte; EC, endothelial cells; FB, fibroblasts; SMC, smooth muscular cell.
