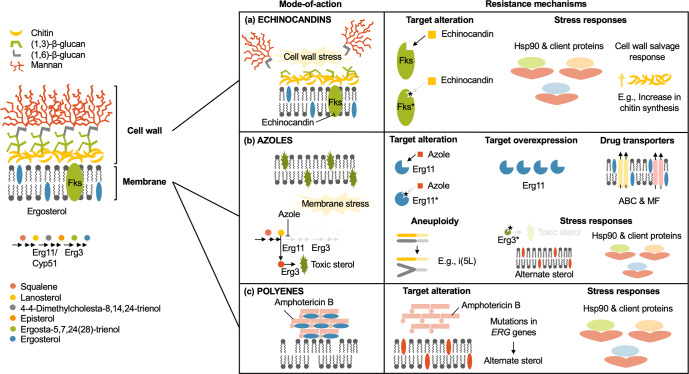
Fig. 1. Antifungal mode-of-action and mechanisms of resistance.
a The echinocandins function by inhibiting (1,3)-β-D-glucan synthase, disrupting cell wall integrity and causing severe cell wall stress. Echinocandin resistance is primarily mediated by mutations in the drug target gene, FKS1, in Candida, Cryptococcus, and Aspergillus. For C. glabrata, mutations occur in both FKS1 and its paralogue FKS2. Cellular factors enabling responses to echinocandin-induced stress include the molecular chaperone Hsp90, various Hsp90 client proteins, and genes that regulate cell wall salvage signalling (e.g., compensatory upregulation of chitin synthesis). b The azoles act on the fungal cell membrane via the inhibition of lanosterol 14-α-demethylase (Erg11), which blocks ergosterol biosynthesis and results in the accumulation of a toxic sterol intermediate (14-α-methyl-3,6-diol) produced by Erg3. Azole resistance can arise through mutations in the drug target (ERG11) or target overexpression. Loss-of-function mutations in ERG3 can also confer azole resistance by blocking toxic sterol accumulation and this mechanism is contingent on Hsp90 and its client proteins. Efflux is also a major azole resistance determinant and involves the upregulation of ABC and MF transporters. Aneuploidies such as a duplication of the left arm of chromosome 5 (termed isochromosome (i5(L))) can increase dosage of the azole target Erg11 and efflux pumps. c The polyene drug amphotericin B forms extramembranous aggregates that extract ergosterol from fungal cell membranes, acting as a sterol “sponge”. While resistance remains extremely rare, it can be acquired through mutations in ergosterol biosynthesis genes resulting in the depletion of ergosterol and the accumulation of alternate sterols. As with resistance to other antifungals, resistance to amphotericin B is also contingent on Hsp90-dependent stress responses.