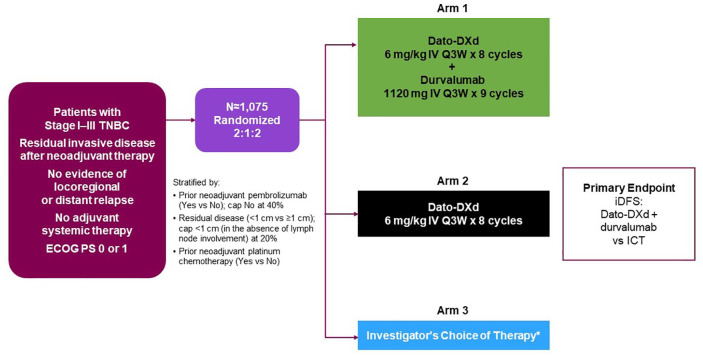
Figure 1.
TROPION-Breast03 study design.
*Capecitabine (1000 or 1250 mg/m2 BID for eight cycles) and/or pembrolizumab (200 mg IV Q3W for nine cycles); only patients who have received prior pembrolizumab in the neoadjuvant setting should receive pembrolizumab as part of their adjuvant therapy in the Investigator’s Choice of Therapy arm.
BID, twice per day; Dato-DXd, datopotamab deruxtecan; ECOG PS, Eastern Cooperative Oncology Group performance status; ICT, investigator’s choice of therapy; iDFS, invasive disease-free survival; IV, intravenous; Q3W, every 3 weeks; TNBC, triple-negative breast cancer.