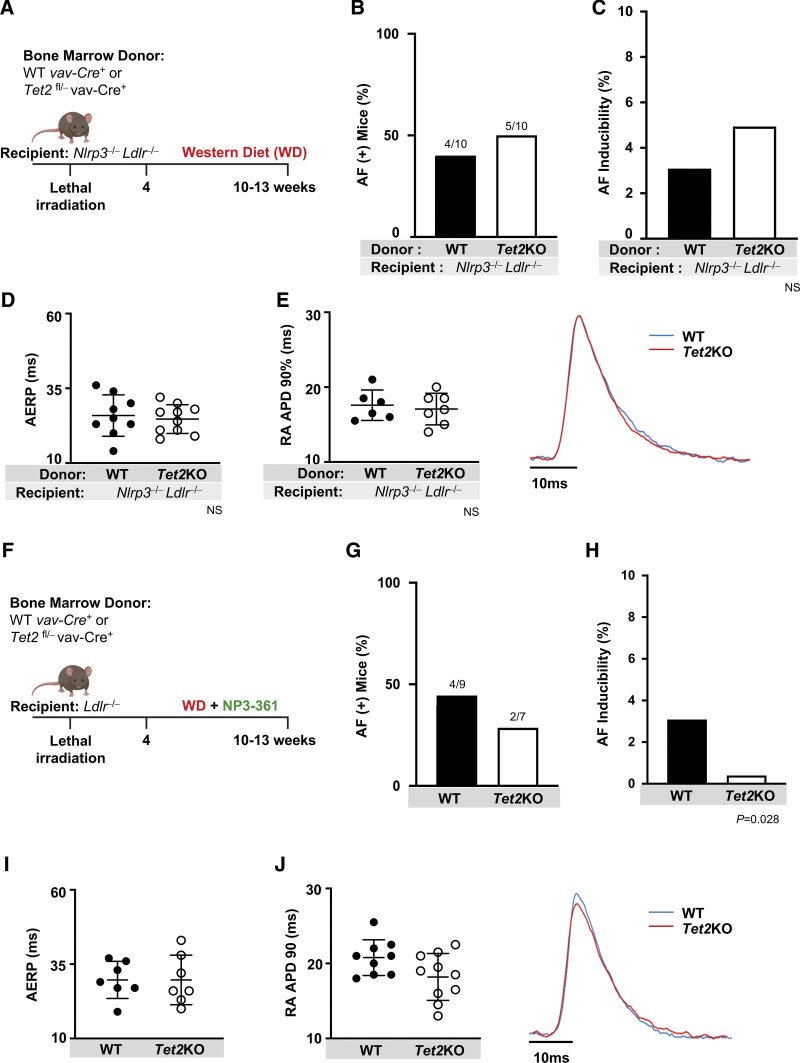
Figure 3.
NLRP3 inflammasome activation is required for enhanced AF inducibility stemming from hematopoietic-specific Tet2 deficiency. A, Lethally irradiated Ldlr−/− Nlrp3−/− mice were transplanted with bone marrow with hematopoietic-specific inactivation of Tet2 (Tet2KO) or WT controls and then fed WD for a total of 6 to 9 weeks. B, Hematopoietic-specific Tet2 inactivation did not affect number of mice with AF (number of mice with AF/total mice in the group) or C, AF inducibility in Ldlr−/− Nlrp3−/− mice. D, NLRP3 deficiency abrogated Tet2KO-related shortening in AERP as well as E, RA APD90. Representative tracing (right). F, Lethally irradiated Ldlr−/− mice were transplanted with bone marrow with hematopoietic-specific inactivation of Tet2 (Tet2KO) or WT controls and then fed WD with an incorporated NLRP3 inhibitor, NP3-361, for a total of 6 to 9 weeks. G, Hematopoietic-specific Tet2 deficiency did not increase number of mice with AF, nor H, AF inducibility in Ldlr−/− mice treated with NP3-361. I, NP3-361 treatment abrogated Tet2KO-related shortening in AERP as well as J, RA APD90. Representative tracing (right). Statistical testing: Fisher exact test for B, C, G, and H; 2-tailed Student t test and mean (SD) shown for D, E, I, and J. AERP indicates atrial effective refractory period; AF, atrial fibrillation; KO, knockout; LDLR, low-density lipoprotein receptor; NLRP3, NLR (NACHT, LRR [leucine rich repeat]) family pyrin domain containing protein 3; RA APD90, right atrial action potential duration at 90% repolarization; TET2, tet methylcystosine dioxygenase 2; and WD, Western diet; and WT, wild-type.