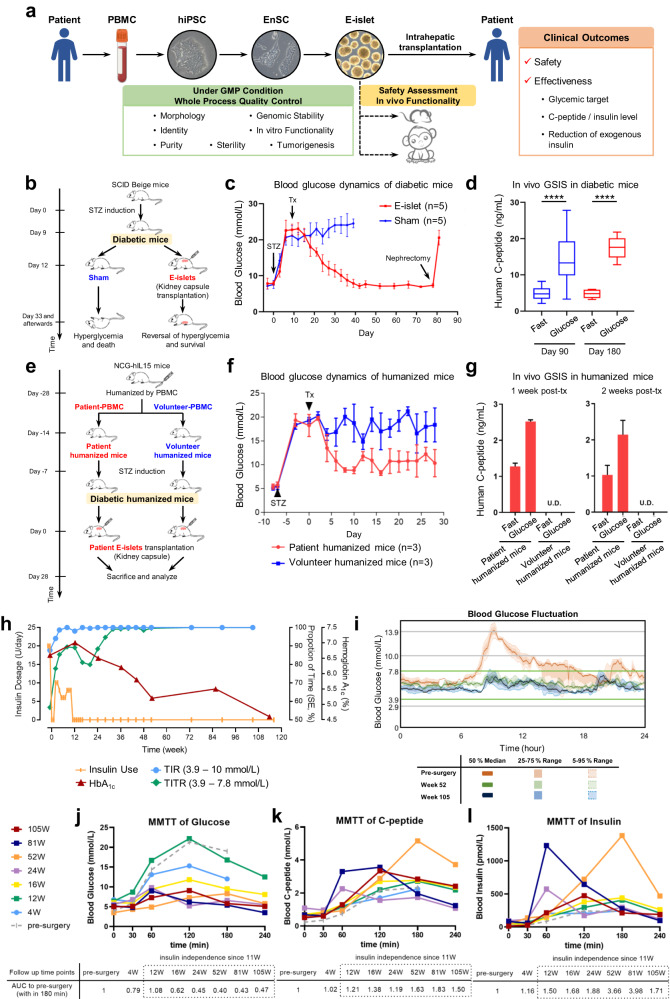
Fig. 1. Preclinical studies and clinical outcomes of autologous E-islet transplantation in a T2D patient.
a Brief scheme of major procedures involved in the generation and quality control of E-islets and the safety/effectiveness evaluations of E-islet transplantation. b–d E-islets reverse hyperglycemia in STZ-induced diabetic immunocompromised mice. Schematic illustration of kidney capsule transplantation of E-islets (b). Fasting blood glucose dynamics (blue line: sham group; red line: E-islet-transplanted group, c). Secretion of human C-peptide after fasting and 30 min following an i.p. glucose bolus on days 90 and 180 post transplantation (d). e–g Immunogenicity of E-islets in humanized mice. Schematic illustration of the syngeneic and allogeneic kidney capsule transplantation of patient-specific E-islets into the NCG-hIL15 diabetic mice humanized with the patient’s and a volunteer’s PBMCs (e). Fasting blood glucose dynamics (blue line represents the control group with the patient E-islets transplanted into three diabetic mice humanized with the volunteer’s PBMCs; red line represents the group with the patient E-islets transplanted into three diabetic mice humanized with the patient’s PBMCs, f). Secretion of human C-peptide after fasting and 30 min following an i.p. glucose bolus on days 7 and 14 post E-islet transplantation (U.D. undetectable, g). h Clinical measurements of TITR, TIR and HbA1c, and the insulin dosage during 116 weeks. i Continuous interstitial glucose fluctuations derived from the CGM measurements at weeks 52 and 105 compared with pre-surgery levels. j–l Serum levels of fasting and meal-stimulated circulating glucose (j), C-peptide (k) and insulin (l) from MMTT assays.