Figure 2. Estimated Mean Change From Baseline for Gantenerumab, Solanezumab, and Placebo for Cerebrospinal Fluid (CSF) and Plasma Markers.
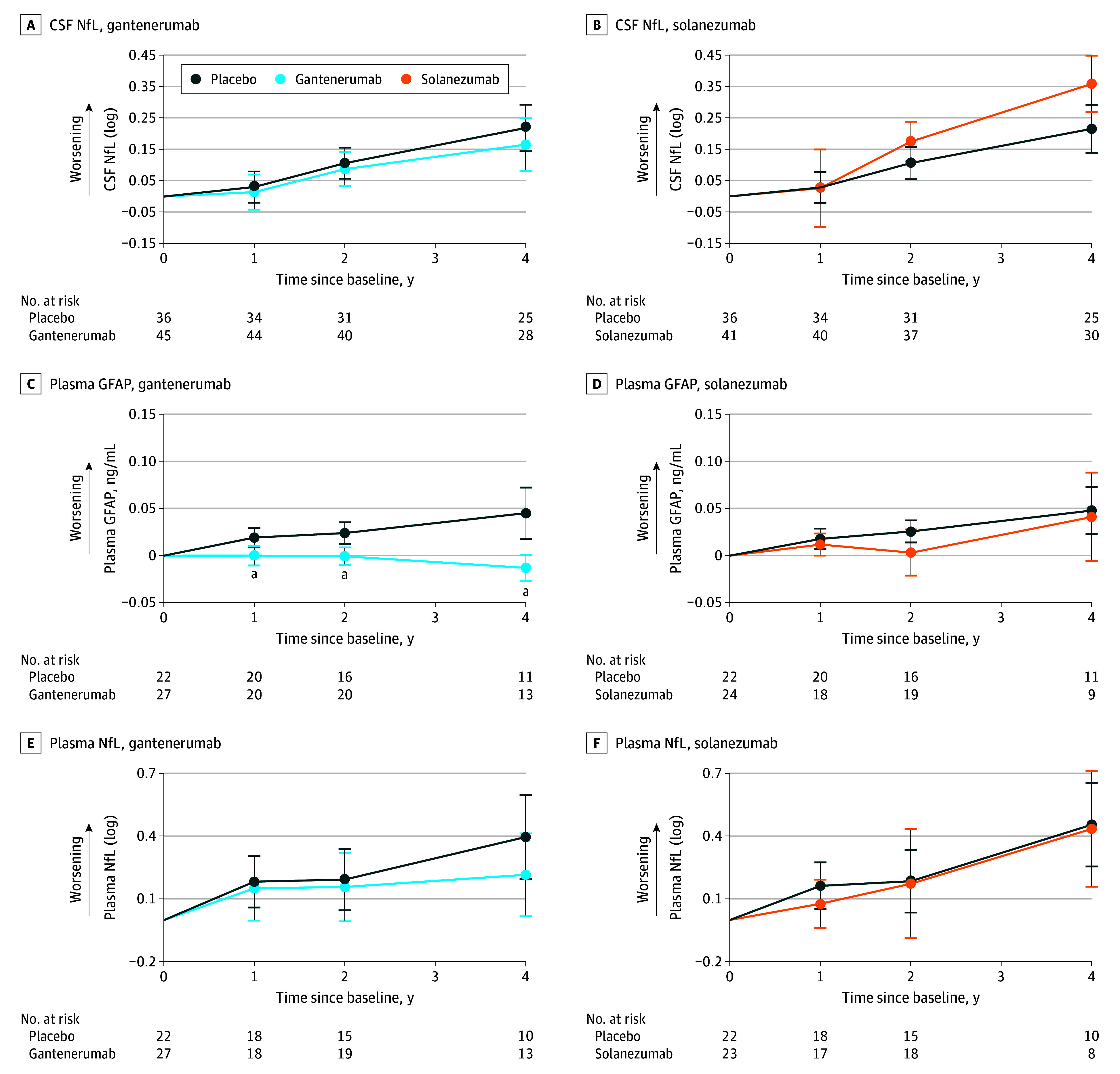
Assessment of CSF markers was done for both gantenerumab and solanezumab, respectively, in neurofilament light protein (NfL; A and B) and of plasma markers in glial fibrillary acidic protein (GFAP; C and D) and NfL (E and F). All estimations are shown with 95% CI error bars.
aResembles a significance of a P value <.05 or lower (Table 2).
