Figure 2.
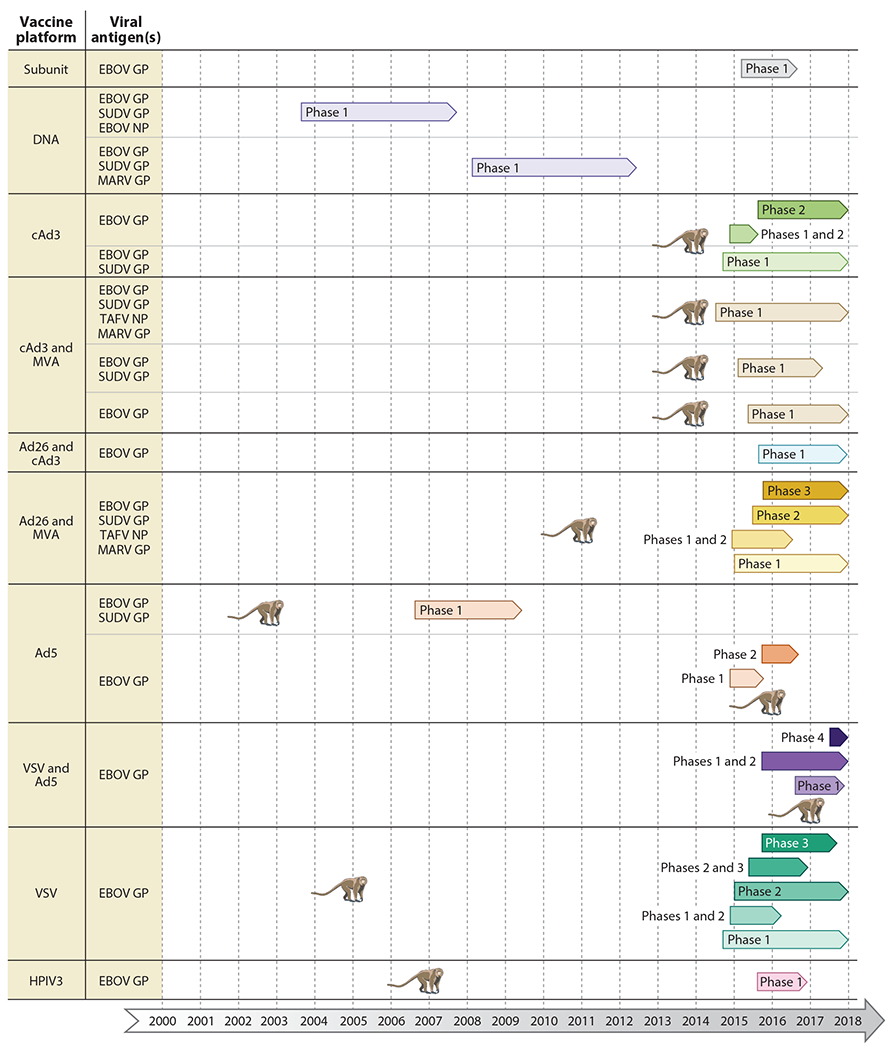
Vaccine platforms in clinical trials. Completed and ongoing phase 1–4 clinical trials are shown for each vaccine platform, with various antigen combinations. The monkey symbolizes the first publication demonstrating complete protective efficacy of the vaccine approach in nonhuman primates. Abbreviations: Ad, human adenovirus; cAd, chimpanzee adenovirus; GP, glycoprotein; HPIV3, human parainfluenza virus type 3; MARV, Marburg virus; MVA, modified vaccinia virus Ankara; NP, nucleoprotein; SUDV, Sudan virus; TAFV, Taï Forest virus; VSV, vesicular stomatitis virus.
