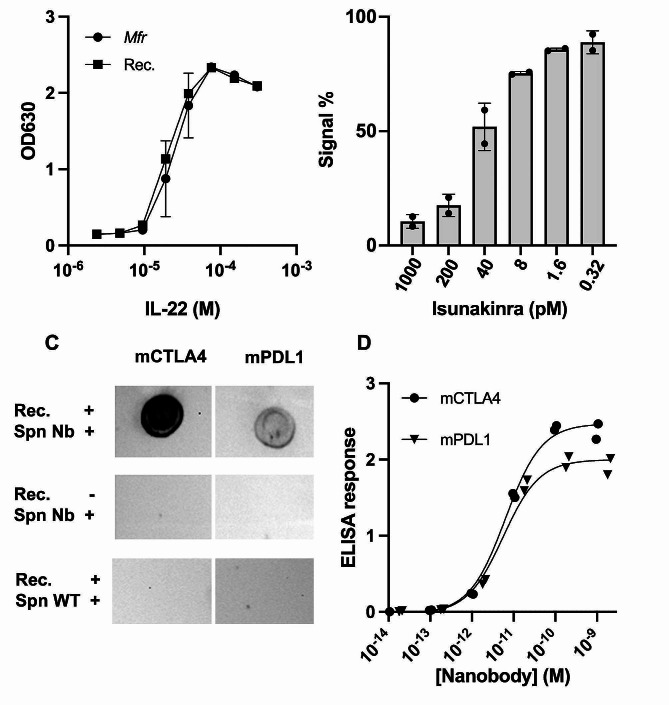
Fig. 4.
Validation of Mfr platform secretion of clinically relevant active biomolecules. (A) HekBlue reporter activation measured by absorbance 630 (OD630) of hIL-22 secreted by Mfr (circle) compared to commercially recombinant protein produced in E.coli (square). Data shown as average +/- SD of two biological replicates with two technical replicas (N = 2). (B) Antagonist response of increasing concentrations of Isunakinra produced in Mfr against HekBlue cell lines stimulated with 9 pM of recombinant IL-1β. Data shown as average +/- SD of two biological replicates with two technical replicates (N = 2). (C) Dotblot assay measuring the binding of nanobodies produced in Mfr against murine CTLA4 and PDL1. Rec. + indicates the addition of the recombinant protein. Spn Nb refers to the addition of the respective nanobody. Spn WT indicates where supernatant of WT protein was added to check for unspecific background to the target protein. The exposure time was 4 min. and 21 s. (D) ELISA assay measuring the binding of murine nanobodies to their target. ELISA response refers to the optical density at 450 nm subtracted to the one at 560 nm. Data is shown with each biological replicate individually with a calculated curve for each nanobody (N = 2)