Abstract
Background:
The optimal treatment regimen for patients with Hughes syndrome remains unclear. Therefore, the authors sought to compare the outcomes of warfarin vs. factor Xa inhibitors in patients with Hughes syndrome.
Methods:
MEDLINE, Embase, and Cochrane Central databases were searched for randomized controlled trials (RCTs) comparing 8 efficacy and safety of warfarin and factor Xa inhibitors in patients with Hughes syndrome. Recurrent thrombosis, all-cause mortality, stroke, adverse reactions, and bleeding were among 10 outcomes of interest. Mantel–Haenszel weighted random-effects model was used to calculate 11 relative risks (RRs) with 95% CIs.
Results:
The analysis included 625 patients from four RCTs and one post-hoc analysis. Meta-analysis showed a statistically non-significant difference between factor Xa inhibitors and warfarin in the recurrent thrombosis risk (arterial or venous) [RR 2.77 (95%, CI 0.79, 9.65); P=0.11, I2=50%]. Consistent results were revealed among patients with a previous history of arterial thrombosis [RR 2.76 (95% CI 0.93, 8.16); P=0.75, I2=0%], venous thrombosis [RR 1.71 (95% CI 0.60, 4.84); P=0.31, I2=15%] and patients who were triple antiphospholipid antibodies (aPL) positive [RR 4.12 (95% CI 0.46, 37.10); 21 P=0.21, I2=58%]. Factor Xa inhibitors were significantly associated with an increased risk of stroke [RR 8.51 (95% CI 2.35, 13.82); P=0.47, I2=0%].
Conclusion:
Factor Xa inhibitors exhibited an increased risk of stroke among patients with Hughes syndrome. In addition, although not significant, the higher RRs among patients on factor Xa inhibitors may indicate a higher risk of thrombotic events associated with factor Xa inhibitors.
Keywords: factor xa, hughes, meta-analysis, warfarin
Introduction
Highlights
Various anticoagulation regimens have been used for thromboprophylaxis in patients with Hughes syndrome.
Factor Xa inhibitors show similar efficacy in preventing the risk of recurrent thrombosis.
However, compared with warfarin, factor Xa inhibitors inhibit increased risk of stroke.
Hughes syndrome is a prevalent form of acquired thrombophilia and is an autoimmune disorder. Patients with Hughes syndrome are distinguished by the existence of circulating antiphospholipid antibodies (aPL), namely lupus anticoagulant (LA), IgG or IgM anticardiolipin (aCL), and IgG or IgM anti-2glycoprotein-I (a2-GPI)1. Additionally, they must meet at least one clinical criterion, which involves obstetrical morbidity and/or venous or arterial thrombosis2. Given that thrombosis is the leading cause of morbidity and mortality in this population, long-term anticoagulation is advised as a secondary thromboprophylaxis measure. Despite remaining the fundamental agent in thrombotic management, warfarin’s restricted therapeutic index requires frequent dose modifications and international normalized ratio (INR) monitoring3. Moreover, these patients encounter challenges in maintaining long-term therapy, particularly due to the significant risk of major bleeding that occurs when the INR falls below the therapeutic range of 2.0–3.0. Although factor Xa inhibitors have a reduced likelihood of substantial bleeding, a fixed dosage, and fewer drug-food interactions, they have gained popularity as an alternative thromboprophylaxis strategy in recent times4,5.
Prior to recent years, the majority of the evidence supporting optimal anticoagulation for patients with Hughes syndrome came from small-power cohort studies, case reports, and case series6–9. In pivotal trials, more recent randomized controlled trials (RCTs) that compare warfarin and factor Xa inhibitors in patients with Hughes syndrome have enabled the derivation of a valid conclusion regarding the safety and efficacy of these anticoagulant regimens. Prior research contrasted warfarin and factor Xa inhibitors in aPL-positive patients using comparable studies in a meta-analysis10–13. However, it was deficient in adverse outcome assessment and sensitivity analysis, and it included cohort studies susceptible to confounding bias. Furthermore, their failure to integrate the revolutionary findings of the recently concluded ASTRO-APS study necessitated a revised evaluation and meta-analysis14. Comparing the safety and efficacy of factor Xa inhibitors and warfarin was thus the objective of this study, which sought to determine which was the most secure and effective treatment option for these patients.
Methods
This meta-analysis adhered to the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidelines15, Supplemental Digital Content 1, http://links.lww.com/MS9/A421 and the Risk of Bias in Systematic reviews and assessment of multiple systematic reviews (AMSTAR) 216, Supplemental Digital Content 2, http://links.lww.com/MS9/A422. The study in question is registered in the International Prospective Register of Systematic Reviews (PROSPERO), which is overseen by the National Institute for Health Research (NIHR).
As the material was publicly available, there was no need for permission from the institutional review board (IRB).
Data sources and search strategy
The databases MEDLINE, EMBASE, and Cochrane CENTRAL were thoroughly searched by two independent reviewers (B.B.S. and A.S.) from their creation until January 2024. We selected research based on the content of their abstracts and titles. A comprehensive evaluation of the text was requested when necessary. The search utilized MeSH terms and keywords to identify both generic and brand names of anticoagulant drugs, as well as symptoms associated with Hughes Syndrome. The entire search technique for both databases may be seen in Supplemental Table 1, Supplemental Digital Content 3, http://links.lww.com/MS9/A423.
Study selection
We considered studies that met the following criteria: (1) they were randomized controlled trials (RCTs) or involved the analysis of RCTs, and (2) they compared factor Xa inhibitors and warfarin in separate interventional arms. (2) documented any occurrence of thrombotic or bleeding incidents, any unfavourable incidents or reactions, including mortality from any cause, (3) included patients who tested positive for LA, IgG or IgM aCL, and IgG or IgM anti-2-glycoprotein-I (a2-GPI) antibodies, indicating the presence of at least one persistently positive aPL. In the event of any discrepancies in the research selection process, a third investigator (VK) was engaged for consultation. The articles were subsequently submitted to the Endnote Reference Library (Version X7.5; Clarivate Analytics) software in order to eliminate any duplicate entries.
Data extraction and assessment of study quality
Two reviewers (B.B.S. and A.S.) autonomously retrieved data from the chosen studies, encompassing study characteristics, patient demographics, summary events, event counts, sample sizes, and therapy type. Additionally, relevant outcomes were identified, and risk ratios (RRs) with 95% CIs were computed based on the extracted summary events. We additionally obtained data on the number of participants who had previously had thrombosis, the presence of baseline aPL triple positive, the year of publication, the duration of follow-up, and the average/median ages. The Cochrane Risk of Bias Tool (CRBT) was used to assess the quality of studies in six categories: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias17.
Statistical analysis
The statistical calculations were performed using RevMan (version 5.3; Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration). We employed the Mantel–Haenszel weighted random-effects model to calculate relative risks (RRs) with 95% CI for our statistical analysis. This model was chosen considering the potential variability across the included studies, and it allows for a more conservative estimation of the treatment effects, particularly in the presence of heterogeneity. Heterogeneity across studies was evaluated using the Higgins I2 statistic, which yielded a value of 16. In order to reduce the potential for bias, recurrent thrombosis occurrences were categorized into subgroups according to the specific type of blood vessel affected, whether it was a vein or an artery. Given the limited quantity of research conducted, we refrained from assessing any potential bias in publication.
Results
Literature search and characteristics of included studies
The PRISMA flow diagrams provide a depiction of the process used to conduct a literature search and identify relevant research studies (Fig. 1). Out of the initial 1137 papers, only 4 RCTs and 1 post-hoc analysis were selected for this analysis. These studies included a total of 625 patients. Table 1 presents the demographic and baseline information. The average ages of the patients ranged from 46 to 51. The proportion of women varied from 62 to 84% among the trials.
Figure 1.
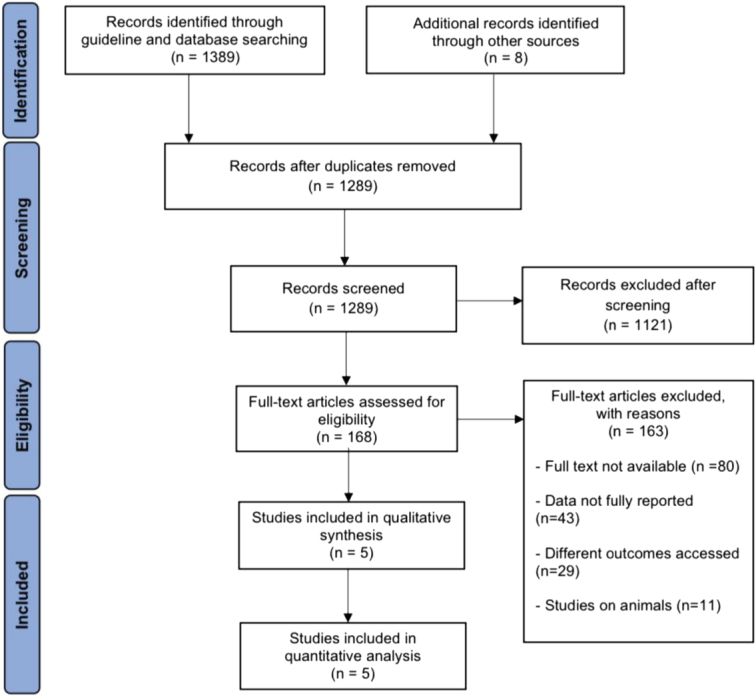
PRISMA flow diagram of study identification for meta-analysis.
Table 1.
Baseline demographics and study characteristics of included studies
| First author, year (study name) | Study design | Total study population | N (factor Xa) | N (warfarin) | Factor Xa inhibitors used | Female sex (%) (factor Xa vs. warfarin) | Age (factor Xa vs. warfarin) | Follow-up (in days) | aPL triple positivity (%) | Primary efficacy outcome of thrombosis % (factor Xa vs. warfarin) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cohen, 201610 | RCT | 116 | 57 | 59 | Rivaroxaban | 74% vs. 71% | Mean (SD) =47 (17) vs. 50 (14) | 210 | 25 | 0% vs. 0% |
| Pengo, 201812 | RCT | 120 | 59 | 61 | Rivaroxaban | 66% vs. 62% | Mean (SD) =47 (10) vs. 46 (13) | 611 | 100 | 22% vs. 3% |
| Ordi-Ros, 201911 | RCT | 190 | 95 | 95 | Rivaroxaban | 67% vs. 72% | Mean (SD) = 47 (7) vs. 51 (13) | 1077 | 61.10 | 12.6% vs. 6.3% |
| Goldhaber, 201613 | Post-hoc analysis | 151 | 71 | 80 | Dabigatran | NR | Mean (SD)=48 (15) vs. 47 (17) | NR | NR | 4.2% vs. 5% |
| Woller, 202218 | RCT | 48 | 23 | 25 | Apixaban | 82.6% vs. 84.0% | Mean (SD)=46 (12) vs. 48.5 (14) | 365 | 29.20 | 26.0% vs. 0% |
aPL, antiphospholipid; N, Number; NR, not reported; RCT, randomized controlled trial.
Recurrent thrombosis
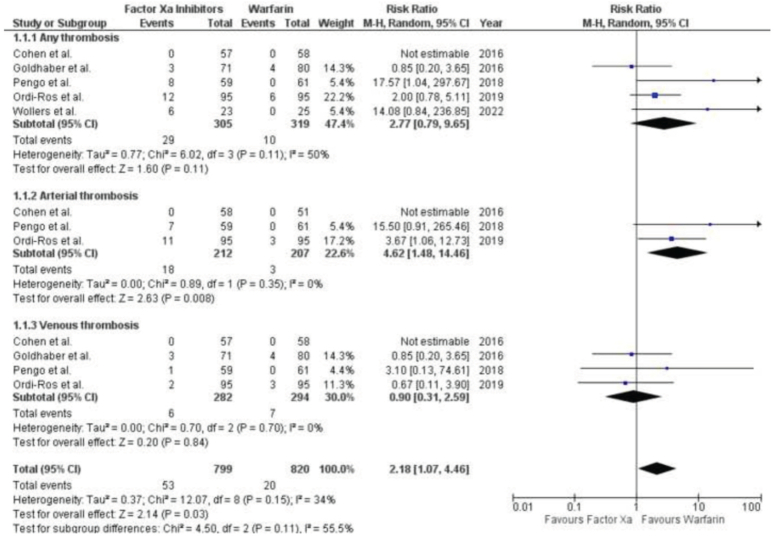
The outcome of recurrent thrombosis was reported in five investigations, consisting of four randomized controlled trials (RCTs) and one post-hoc study. ( Fig. 2 ) The efficacy of factor Xa inhibitors and warfarin in preventing thrombosis did not differ significantly [RR 2.77 (95% CI 0.79, 9.65); P=0.11, I2=50%]. After analyzing subgroups based on the type of thrombosis (venous or arterial), we found that factor Xa inhibitors were significantly associated with an increased risk of arterial thrombosis [RR 4.62 (95% CI 1.48, 14.46); P=0.008, I2=0%]. However, there was no significant difference in the risk of venous thrombosis between factor Xa inhibitors and warfarin [RR 0.90 (95% CI 0.31, 2.59); P=0.70, I2=0%].
Figure 2.
Forest Plot showing the results of Factor Xa Inhibitors Versus Warfarin on Recurrent thrombosis.
Recurrent thrombosis in patients with a predisposition to prior thrombosis or triple aPL positivity
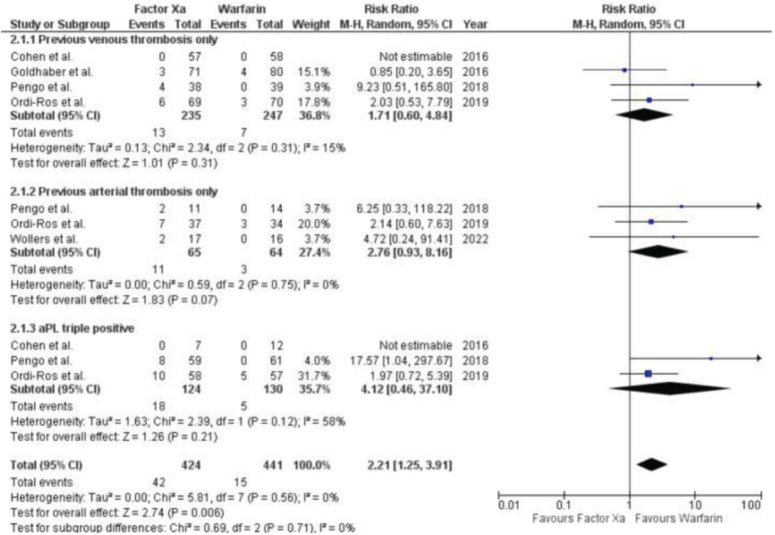
Five studies, including 4 RCTs and 1 post-hoc study, provided information on the occurrence of thrombotic events in patients who had a history of such events and were also positive for triple aPL (Fig. 3). A pooled analysis found that there was no significant difference between patients who had a history of arterial thrombosis and those who had a history of venous thrombosis in terms of their response to treatment with factor Xa inhibitors or warfarin. The relative risk (RR) for arterial thrombosis was 2.76 (95% CI 0.93, 8.16; P=0.75, I2=0%), while the RR for venous thrombosis was 1.71 (95% CI 0.60, 4.84; P=0.31, I2=15%), and the formation of blood clots within veins. In people who tested positive for triple aPL, the use of factor Xa inhibitors did not significantly increase the risk of recurrent thrombosis when compared to warfarin.
Figure 3.
Forest Plot showing the results of Factor Xa Inhibitors Versus Warfarin on Recurrent thrombosis in patients with a predisposition to prior thrombosis or triple aPL positivity.
Bleeding
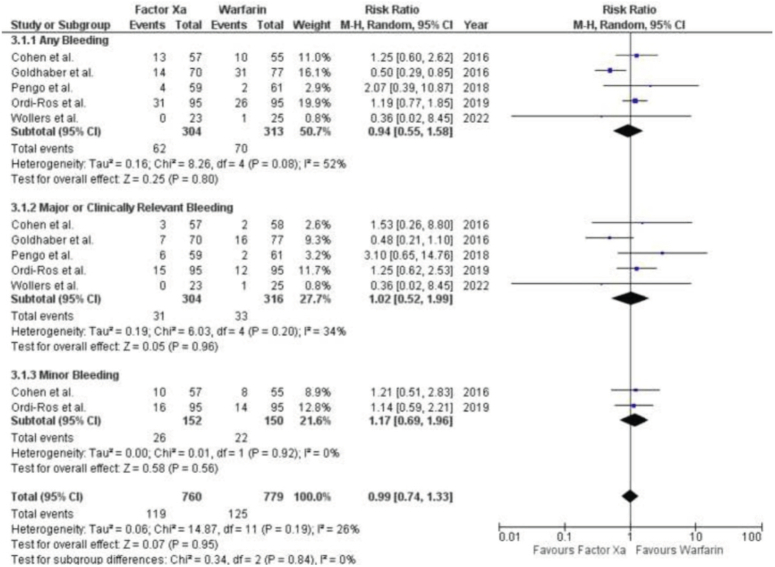
Five research, consisting of four RCTs and one post-hoc study, provided data on bleeding events. There was no significant distinction observed in terms of avoiding bleeding between factor Xa inhibitors and warfarin [RR 0.94 (95% CI 0.55, 1.58); P=0.08, I2=52%]. (Fig. 4) Subgroup analysis revealed no significant differences in the occurrence of major or clinically significant bleeding between Factor Xa inhibitors and warfarin. The results of the study showed a RR of 1.02 with a 95% CI ranging from 0.52 to 1.99. The P value was 0.96, indicating no significant difference. The I2 value was 34%, suggesting moderate heterogeneity.
Figure 4.
Forest Plot showing the results of Factor Xa Inhibitors Versus Warfarin on Bleeding.
Adverse events or reactions
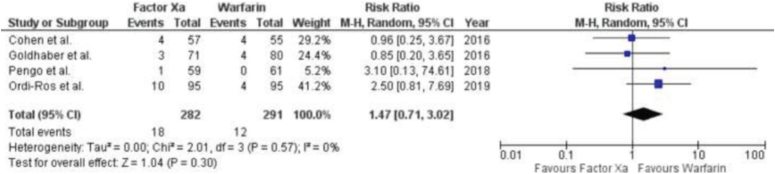
Four investigations, consisting of three RCTs and one post-hoc study, documented distinct adverse occurrences or reactions (Fig. 5). No significant disparity was observed between factor Xa inhibitors and warfarin in terms of bleeding. The results of the study showed a RR of 1.47 with a 95% CI ranging from 0.71 to 3.02. The P value was 0.30, indicating that there was no statistically significant difference. The I2 statistic was 0%, suggesting no heterogeneity among the studies.
Figure 5.
Forest Plot showing the results of Factor Xa Inhibitors Versus Warfarin on Adverse events or reactions.
Cerebrovascular accident
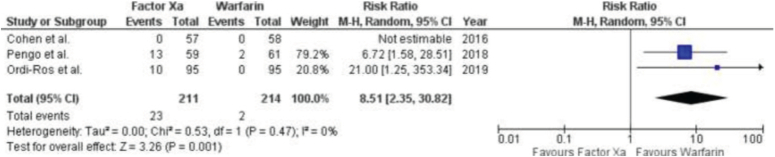
Three RCTs presented results about stroke (Fig. 6). The meta-analysis revealed that patients who were prescribed factor Xa inhibitors had a significantly greater likelihood of experiencing a stroke compared to those who were taking warfarin. The RR was 8.51 (95% CI: 2.35–13.82), with a P value of 0.47 and an I2 value of 0%.
Figure 6.
Forest Plot showing the results of Factor Xa Inhibitors Versus Warfarin on Cerebrovascular accident.
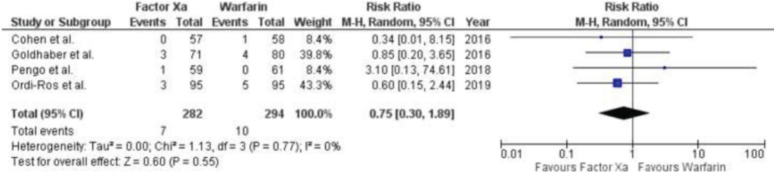
All-cause mortality
Four RCTs presented results on stroke, as shown in Figure 7. A meta-analysis revealed no significant disparity in the risk of all-cause mortality between persons treated with factor Xa inhibitors and warfarin. The RR is 0.75 with a 95% CI of 0.30–1.89. The P value is 0.55 and the I2 statistic is 0%.
Figure 7.
Forest Plot showing the results of Factor Xa Inhibitors Versus Warfarin on All-cause mortality.
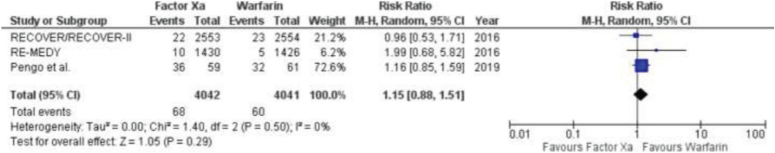
Pulmonary embolism
Two studies provided data on pulmonary embolism (Fig. 8). A meta-analysis revealed no significant disparity in the incidence of pulmonary embolism between those taking factor Xa inhibitors and warfarin. The results showed a risk ratio of 1.15 with a 95% CI ranging from 0.88 to 1.51. The P value was 0.29, indicating no statistically significant difference. The I2 value was 0%, suggesting no heterogeneity among the studies.
Figure 8.
Forest Plot showing the results of Factor Xa Inhibitors Versus Warfarin on Pulmonary embolism.
Quality assessment
Based on the Cochrane risk-of-bias approach for randomized trials, the RCTs were assessed to have a moderate risk of bias. (Figure S1, Supplemental Digital Content 3, http://links.lww.com/MS9/A423).
Discussion
This study presents a comparison of safety and efficacy outcomes between warfarin and factor Xa inhibitors in patients with Hughes syndrome, revealing some significant findings. Initially, it was discovered that Xa inhibitors were strongly linked to a heightened risk of recurring arterial thrombosis. Furthermore, individuals receiving factor Xa inhibitors exhibited a higher propensity for stroke in comparison to those using warfarin. Furthermore, individuals who had previously suffered from arterial thrombosis were markedly more prone to encountering arterial thrombosis again. Furthermore, the utilization of factor Xa inhibitors did not result in an elevated risk of all-cause mortality, adverse events, or bleeding (regardless of severity) when compared to warfarin.
With the exception of end-stage renal illness, factor Xa inhibitors have a proven safety and efficacy profile that is at least as good as warfarin in several patient situations, including non-valvular atrial fibrillation (NVAF) and end-stage renal disease (ESRD)19–22. Nevertheless, there is ongoing controversy regarding the most effective anticoagulant regimen for patients with Hughes Syndrome, particularly those at high risk. The lack of greater effectiveness and safety of factor Xa inhibitors compared to warfarin in patients with Hughes syndrome may be attributed to the variability observed in the research included. The eligibility criterion for TRAPS was limited to patients who tested positive for all three anti phospholipid antibodies (triple-positive aPL patients). A significant majority of the patients included in the study conducted by Ordi-Ros and colleagues had previously experienced arterial thrombosis. This means that the effectiveness of factor Xa inhibitors in a specific group of low-risk patients was not evaluated, potentially undermining the ability of factor Xa inhibitors to prevent recurrent thrombosis in these patients.
Patients diagnosed with Hughes syndrome are treated for acute thrombotic events using the same approach as the general population. Patients necessitate warfarin administration, followed by heparin, for the purpose of managing anticoagulation. Long-term oral anticoagulation is the most effective preventive therapy against recurrent thrombosis, surpassing the effectiveness of antiaggregant medications. This treatment may need to be continued indefinitely. The optimal therapeutic INR is still a subject of ongoing debate. Although the results did not achieve statistical significance, the relative risks (RRs) for venous thromboses were quite similar in both groups. These findings corroborate previous studies that examined the same result and suggest that factor Xa inhibitors can be used safely in low-risk patients who are at risk of venous thromboembolism23. The lack of consistent results in the outcomes of venous thromboses can be attributed to multiple factors and can be explained by several potential explanations. Perzborn et al. 24‘s research indicates that rivaroxaban demonstrates higher antithrombotic efficacy in the venous model compared to the arterial model. Furthermore, the limited compliance with factor Xa inhibitors may be ascribed to their ineffectiveness in patients with Hughes syndrome. Nevertheless, the trials included in our study documented adherence rates exceeding 95%. Additionally, the limited number of participants in the study may have hindered the ability of factor Xa inhibitors to effectively demonstrate their effectiveness in reducing illness and death rates. Further clinical studies should be conducted to investigate the efficacy of factor Xa inhibitors, specifically in patients with venous thromboses, due to the differing patterns shown in their performance in avoiding arterial and venous thrombosis. Regrettably, the warnings provided by EMA and MHRA guidelines25 will decrease the probability of conducting future randomized controlled trials (RCTs), resulting in a lack of knowledge to further comprehend the subject.
Two recent trials, namely the Rivaroxaban vs. vitamin K Antagonist in Antiphospholipid Syndrome (EUDRA-2010-019764-36) study done by Ordi-Ros and colleagues and the ASTRO-APS study conducted by Wollers and colleagues, involved a total of 190 participants. Eleven and forty-eight patients, respectively, are participants in the limited number of RCTs investigating the optimal anticoagulation strategy for Hughes syndrome. Our findings indicate that factor Xa inhibitors are less effective than warfarin in terms of both thrombotic events and severe bleeding. The significance of these discoveries lies in their potential to directly impact clinical practice by aiding in the decision-making process when selecting the most effective anticoagulant regimen, namely between warfarin and factor Xa inhibitors. However, it is undeniable that the limited range of effective doses for warfarin requires regular adjustments and monitoring of the INR. This adds complexity to the treatment, reduces patient compliance, and worsens the outlook, particularly when patients are already taking medications that are known to impact INR levels. The impact of the LA on particular thromboplastins in individuals with Hughes Syndrome may exacerbate confusion and yield inaccurate INR measurements26,27. While warfarin is often more effective for treating Hughes syndrome, physicians must consider many aspects before making therapy recommendations. Subsequent extensive clinical trials in the future may offer further understanding to determine the most effective anticoagulant treatment for patients with Hughes syndrome.
In addition, we evaluated secondary outcomes in the trials that were included and combined the findings for adverse events and stroke. The definition of adverse events exhibited variability among the studies, and we considered it as a comprehensive outcome encompassing all-cause mortality as well as any occurrence or severe adverse event/reaction. Seventy-five percent of the papers included in the analysis Among the participants aged 9–12, the results of the study showed that the use of warfarin resulted to a notable decrease in stroke cases when the outcome data was combined. Research has demonstrated that arterial thrombosis, which leads to strokes, occurs most commonly in the cerebral vasculature of individuals with Hughes syndrome. This finding confirms the notable association between stroke and arterial thrombosis in our analysis. The ASTRO-APS protocol was revised twice as a result of frequent and recurring thromboses, including strokes, observed in the group of patients receiving apixaban28. However, the higher likelihood of arterial thrombosis and stroke diminishes the feasibility of using factor Xa inhibitors in these individuals, thus compelling doctors to strictly follow guidelines that advocate for the use of warfarin in treating such patients29,30.
There are various constraints to our meta-analysis. Post-hoc analysis of the RE-COVER, RE-COVER II, and RE-MEDY investigations may be affected by various biases due to inadequate statistical power to evaluate the effectiveness of factor Xa inhibitors compared to warfarin in patients with Hughes syndrome31. Secondly, there are limitations specific to each study. Firstly, the TRAPS study had to be terminated early because there were too many thrombotic events in the arm using factor Xa inhibitors. As a result, the trial did not reach its intended sample size. Secondly, the RAPS study had a lower number of high-risk antibodies and a shorter follow-up period, which may explain the absence of thromboembolic events in both arms. Lastly, in Goldhaber’s post-hoc analysis, it was found that the diagnosis for aPL did not fully meet the criteria set by the Sapporo-Sydney guidelines in three randomized controlled trials. Furthermore, we were unable to evaluate the influence of race on the outcomes of warfarin treatment. Lastly, since our research relied on study-level data instead of more dependable individual patient-level data, the results may be susceptible to biased assessments.
Conclusion
The current research examining the use of optimum anticoagulation in Hughes syndrome indicates an increased risk of stroke when undergoing treatment with factor Xa inhibitors. Consequently, factor Xa inhibitors are not a viable substitute for people with Hughes syndrome, particularly those who are at a heightened risk. Further extensive clinical trials, including individuals with relatively low risk, are necessary to gain a better understanding of the effects of factor Xa inhibitors and establish an anticoagulant regimen that is superior in terms of clinical outcomes.
Ethical approval
Not required because data were publicly available.
Consent
Not required because data were publicly available.
Sources of funding
Not applicable.
Author contribution
A.M., A.A., S.M., D.D., M.D. and D.P. conceived the idea and designed the study. O.K. and A.A. collected the data and analyzed it. All authors drafted the manuscript. M.M.K. and S.S. conducted literature search and created the illustrations. A.Q., H.A., U.E. and S.J. revised the manuscript critically.
Conflicts of interest disclosure
Not applicable.
Research registration unique identifying number (UIN)
Name of the registry: NA National Institute for Health Research (NIHR) International prospective register of systematic reviews (PROSPERO).
Unique Identifying number or registration ID: (Identification No. CRD42022356229).
Hyperlink to your specific registration (must be publicly accessible and will be checked). https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=356229.
Guarantor
Sayed Jawad.
Data availability statement
All data used in this article are publicly available in the included studies cited within this mansucript and can be provided upon request.
Provenance and peer review
Not commissioned, externally peer reviewed.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/annals-of-medicine-and-surgery.
Published online 9 April 2024
Contributor Information
Ali Mohtashim, Email: alimohtashim12345@gmail.com.
Aima Azhar, Email: aimaazhar19@gmail.com.
Saad Mazhar, Email: amazharsgd@hotmail.com.
Deepa Devi, Email: Deepadevi99@gmail.com.
Muhammad Danial, Email: daniyalahmed.413@gmail.com.
Dhruvilkumar Patel, Email: dhruvil9965@gmail.com.
Owais Khan, Email: owaiskhan75@hotmail.com.
Anushka Andani, Email: Vanushkaandani13@gmail.com.
Muhammad Mohib Khan, Email: muhammadmohib2000@gmail.com.
Shahzaib Samad, Email: shahzaibsamadshk@gmail.com.
Aena Qureshi, Email: enaqureshi.233@gmail.com.
Hafsa Ali, Email: Hafsaalis12345678@gmail.com.
Umer Ejaz, Email: omerejazf@gmail.com.
Sayed Jawad, Email: SayedJawad12345@outlook.com.
References
- 1.Horstman LL, Jy W, Bidot CJ, et al. Antiphospholipid antibodies: paradigm in transition. J Neuroinflammation 2009;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antovic A, Sennström M, Bremme K, et al. Obstetric antiphospholipid syndrome. Lupus Sci Med 2018;5:e000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribic C, Crowther M. Thrombosis and anticoagulation in the setting of renal or liver disease. Hematol Am Soc Hematol Educ Program 2016;2016:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimmel SE. Warfarin therapy: in need of improvement after all these years. Expert Opin Pharmacother 2008;9:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shantsila E, Lip GYH. Non-Vitamin K Antagonist Oral Anticoagulants: a Concise Guide [Internet]. Cham (CH): Adis; 2016. Chapter 3, Factor Xa Inhibitors. https://www.ncbi.nlm.nih.gov/books/NBK500201/ [PubMed]
- 6.Pastori D, Menichelli D, Cammisotto V, et al. Use of direct oral anticoagulants in patients with antiphospholipid syndrome: a systematic review and comparison of the international guidelines. Front Cardiovasc Med 2021;8:715878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen H Hunt BJ Efthymiou M et al. RAPS trial investigators . Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol 2016;3:e426–e436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Irastorza G, Khamashta MA, Hughes GR. Antiaggregant and anticoagulant therapy in systemic lupus erythematosus and Hughes’ syndrome. Lupus 2001;10:241–245. [DOI] [PubMed] [Google Scholar]
- 9.Cuadrado MJ. Treatment and monitoring of patients with antiphospholipid antibodies and thrombotic history (hughes syndrome). Curr Rheumatol Rep 2002;4:392–398. [DOI] [PubMed] [Google Scholar]
- 10.Cohen H, Hunt BJ, Efthymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol 2016;3:e426–e436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med 2019;171:685–694. [DOI] [PubMed] [Google Scholar]
- 12.Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018;132:1365–1371. [DOI] [PubMed] [Google Scholar]
- 13.Goldhaber SZ, Eriksson H, Kakkar A, et al. Efficacy of dabigatran versus warfarin in patients with acute venous thromboembolism in the presence of thrombophilia: Findings from RE-COVER®, RE-COVERTM II, and RE-MEDYTM. Vasc Med 2016;21:506–514. [DOI] [PubMed] [Google Scholar]
- 14.Woller SC, Stevens SM, Kaplan DA, et al. Apixaban for the secondary prevention of thrombosis among patients with antiphospholipid syndrome: study rationale and design (ASTRO-APS). Clin Appl Thromb Hemost 2016;22:239–247. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Asemota I, Liu B, et al. AMSTAR 2 appraisal of systematic reviews and meta-analyses in the field of heart failure from high-impact journals. Syst Rev 2022;11:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP Altman DG Gøtzsche PC et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv 2022;6:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urooj F, Kulkarni A, Stapleton D, et al. New oral anticoagulants in nonvalvular atrial fibrillation. Clin Cardiol 2016;39:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdullah HM, Ullah W, Jafar MS, et al. Safety and efficacy of apixaban, rivaroxaban, and warfarin in end-stage renal disease with atrial fibrillation: a systematic review and meta-analysis. Cardiovasc Revasc Med 2021;30:26–32. [DOI] [PubMed] [Google Scholar]
- 21.Emamy M, Zahid T, Ryad R, et al. Efficacy and safety of direct factor Xa inhibitors versus warfarin in prevention of primary and secondary ischemic strokes in non-valvular atrial fibrillation: a literature review. Cureus 2020;12:e9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aursulesei V, Costache II. Anticoagulation in chronic kidney disease: from guidelines to clinical practice. Clin Cardiol 2019;42:774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capecchi M, Abbattista M, Ciavarella A, et al. Anticoagulant therapy in patients with antiphospholipid syndrome. J Clin Med 2022;11:6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939--an oral, direct Factor Xa inhibitor. J Thromb Haemost 2005;3:514–521. [DOI] [PubMed] [Google Scholar]
- 25.Bauersachs R, Langer F, Kalka C, et al. Treatment of the antiphospholipid syndrome with direct oral anticoagulantsPosition statement of German societies. Vasa 2019;48:483–486. [DOI] [PubMed] [Google Scholar]
- 26.Noordermeer T, Urbanus RT, Wong CY, et al. Interference in point-of-care international normalized ratio monitoring in patients with lupus anticoagulant is correlated with anti-β2-glycoprotein I antibody titers. Res Pract Thromb Haemost 2022;7:100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dush A, Erdeljac HP. INR management of an antiphospholipid syndrome patient with point-of-care INR testing. J Pharm Pract 2020;33:390–391. [DOI] [PubMed] [Google Scholar]
- 28.Woller SC, Stevens SM, Kaplan DA, et al. Protocol modification of apixaban for the secondary prevention of thrombosis among patients with antiphospholipid syndrome study. Clin Appl Thromb Hemost 2018;24:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Prefer Adherence 2010;4:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X, Cao S, Yu B, et al. Comparing the efficacy and safety of direct oral anticoagulants versus Vitamin K antagonists in patients with antiphospholipid syndrome: a systematic review and meta-analysis. Blood Coagul Fibrinolysis 2022;33:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342–2352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this article are publicly available in the included studies cited within this mansucript and can be provided upon request.