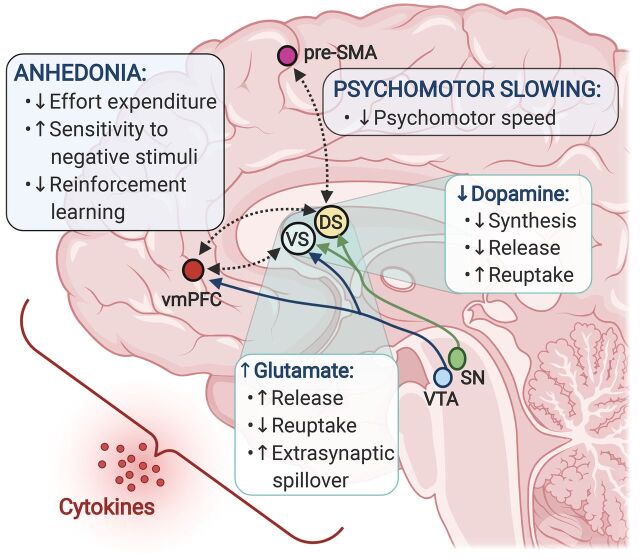
Fig. 1.
Inflammation-induced alterations in dopamine and glutamate converge to affect corticostriatal reward and motor circuitry and drive symptoms of anhedonia and psychomotor retardation. Peripheral innate immune activation and the release of inflammatory cytokines in patients with depression have been associated with elevated glutamate concentrations in basal ganglia regions, as well as decreased dopamine availability and reduced functional connectivity between the ventral and dorsal striatum and reward and motor-related cortical regions, ventromedial prefrontal cortex (vmPFC), and presupplementary motor area (pre-SMA). In turn, inflammation-related changes in both basal ganglia glutamate and corticostriatal connectivity correlated with symptoms of anhedonia and psychomotor retardation and may involve deficits in reward- or goal-directed behaviors such as reward anticipation, effort expenditure, reinforcement learning, and motor control, as well as heightened sensitivity to aversive stimuli. These effects on corticostriatal circuits may be mediated via inflammation-induced decreases in dopamine synthesis and release along with increased reuptake, resulting in overall reduction in dopaminergic signaling. In parallel, inflammatory cytokines promote glutamate release and spillover into the extrasynaptic space while impairing removal of glutamate via reuptake, ultimately contributing to loss of synaptic integrity and circuit dysfunction. DS, dorsal striatum; SN, substantia nigra; VS, ventral striatum; VTA, ventral tegmental area.