Fig. 3.
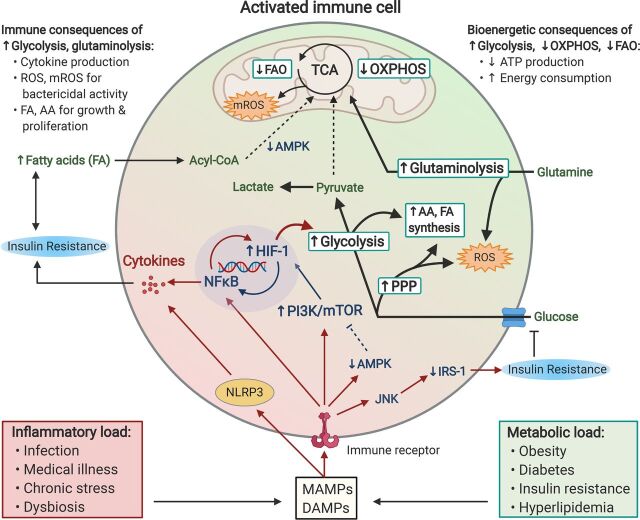
Chronic inflammation and metabolic dysfunction propagate pathologic immunometabolism in activated immune cells, leading to immune and bioenergetic consequences that may serve as a biomarker and pharmacological target for anhedonia. Excess inflammatory and metabolic load due to lifestyle and other factors lead to the release of DAMPs and microbe-associated molecular patterns (MAMPs), which bind to various immune receptors (e.g., cytokine, pattern recognition, and T-cell receptors) on immune cells that in turn activate NFκB and the NLRP3 inflammasome. Activated immune cells subsequently undergo rapid shifts in glucose, lipid, and amino acid metabolism that accommodate their cellular functions, including cytokine production, bactericidal activity, cellular growth, and proliferation. Immune cells primarily derive their energy via a two-step process whereby glucose is first metabolized into pyruvate via glycolysis, a reaction which yields 2 ATP. In resting leukocytes, most of the resulting pyruvate is then shuttled into the citric acid cycle (TCA) to enable OXPHOS, which drives the synthesis of up to 34 ATP per glucose molecule. In contrast, activated immune cells shift to aerobic glycolysis, whereby the majority of pyruvate is converted into lactate, thus dramatically reducing ATP generation via OXPHOS. Crucial to this proglycolytic shift is activation of the transcription factor HIF-1, which commits the cell to glycolysis, thus allowing limited but precipitous energy production. HIF-1 activity is driven by both NFκB transcription as well as DAMP/MAMP-induced activation of the PI3K-mTOR axis. Activated immune cells also rely on an ancillary glycolytic cascade, the pentose phosphate pathway (PPP), glutaminolysis, and the TCA cycle to produce reactive oxygen species (ROS) and mitochondrial ROS (mROS), necessary for their bactericidal actions. Intermediates from glycolysis and PPP additionally provide cellular building blocks [amino acid (AA), fatty acid (FA) synthesis] necessary for rapid cell proliferation. DAMPs and MAMPs also suppress the energy sensor AMP-activated protein kinase (AMPK), which promotes fatty acid (Acyl-CoA) transport into the TCA, thus limiting FAO, another major source of ATP. Together, reduced energy production resulting from a shift toward glycolysis and away from OXPHOS and FAO, along with the high energetic cost of chronic low-grade inflammation, creates a bioenergetic demand potentially relevant to anhedonia. This energy demand can be further exacerbated by concurrent metabolic dysfunction such as increased availability of free fatty acids, as well as insulin resistance due to inflammatory signaling via c-Jun N-terminal kinase (JNK) to inhibit insulin receptor substrate-1 (IRS-1) signaling, thus further limiting energy obtained through glycolysis.