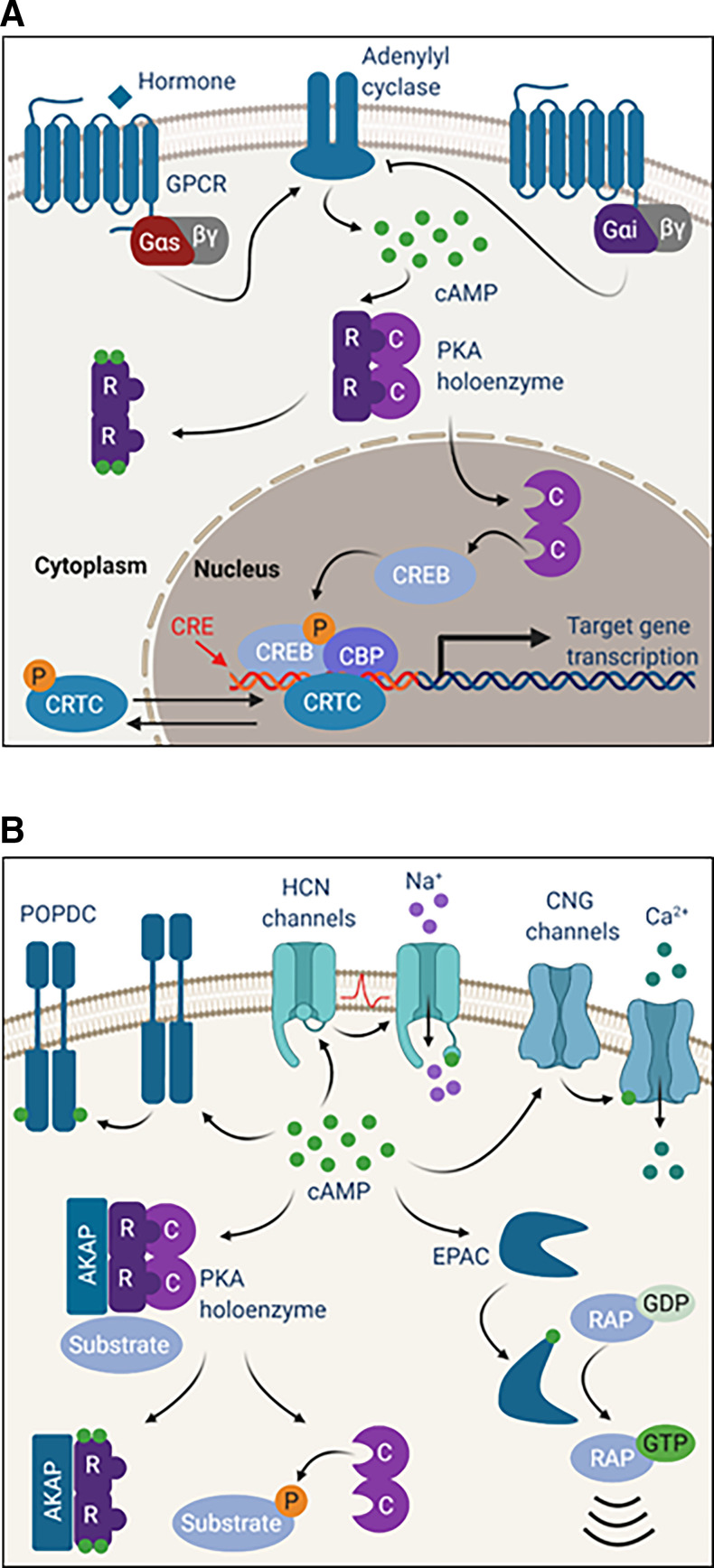
Fig. 3.
(A) Protein kinase A drives CREB-mediated transcription. When hormone binds to Gαs-linked GPCRs on the cell surface, signaling through adenylyl cyclase stimulates cAMP production and PKA activation. Activation of Gαi-coupled GPCRs inhibits adenylyl cyclase and cAMP production. When active, C subunits translocate to the nucleus to phosphorylate CREB on serine 133. Phosphorylated CREB recruits coactivators like CBP to facilitate binding to CREs and transcription of target genes. Additional coactivators, like CRTCs, help to regulate CREB-mediated transcription. Phosphorylation of CRTCs by other kinases results in cytoplasmic sequestration, whereas dephosphorylation by phosphatase enables translocation to the nucleus. (B) cAMP binds and activates effectors beyond PKA. Binding of cAMP to CNG ion channels regulates channel opening and cation currents. HCN channels also bind cAMP to facilitate channel opening by membrane hyperpolarization. cAMP binds to EPAC to facilitate the exchange of GDP for GTP on the RAP family of small GTPases. POPDC proteins reside on the cell surface as dimers that bind cAMP.