Figure 1. agr protects from killing by H2O2 throughout the growth cycle.
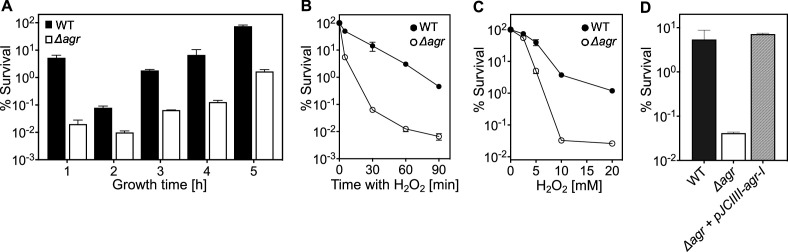
(A) Effect of culture growth phase. Overnight cultures of S. aureus LAC wild-type (WT, BS819) or Δagr (BS1348) were diluted (OD600∼0.05) into fresh TSB medium and grown with shaking from early exponential (1 h, OD600∼0.15) through late log (5 h, OD600∼4) phase. At the indicated times, early (undiluted) and late exponential phase cultures (diluted into fresh Tryptic Soy Broth (TSB) medium to OD600∼0.15) were treated with H2O2 (20 mM). After 60 min, aliquots were removed, serially diluted, and plated for determination of viable counts. Percent survival was calculated relative to a sample taken at the time of H2O2 addition. (B) Kinetics of killing by H2O2. Wild-type and Δagr mutant strains were grown to early exponential (OD600∼0.15) and treated with 20 mM H2O2 for the times indicated, and percent survival was determined by plating. (C) Effect of H2O2 concentration on survival. Cultures prepared as in panel B were treated with the indicated peroxide concentrations for 60 min prior to plating and determination of percent survival. (D) Complementation of agr deletion mutation. Cultures of wild-type (WT) cells (BS819), Δagr mutant (BS1348), and complemented Δagr mutant carrying a chromosomally integrated wild-type operon (pJC1111-agrI) were treated with 20 mM H2O2 for 60 min followed by plating to determine percent survival. Data represent the means ± SD. from biological replicates (n=3).
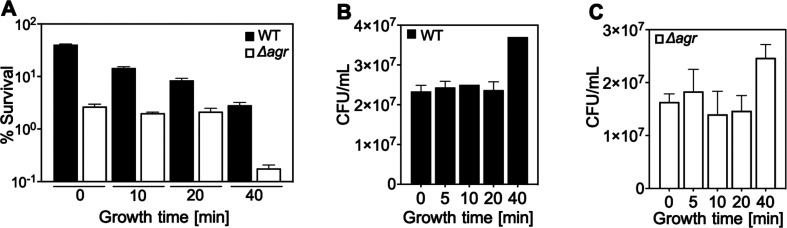
Figure 1—figure supplement 1. Correlation of growth phase and agr expression.
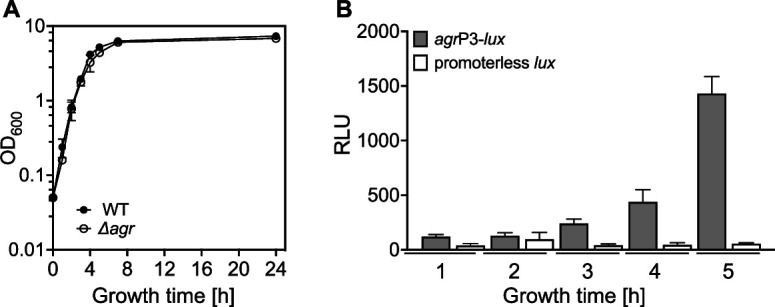
Figure 1—figure supplement 2. Correlation of lag-time and agr-mediated protection from H2O2-mediated killing.
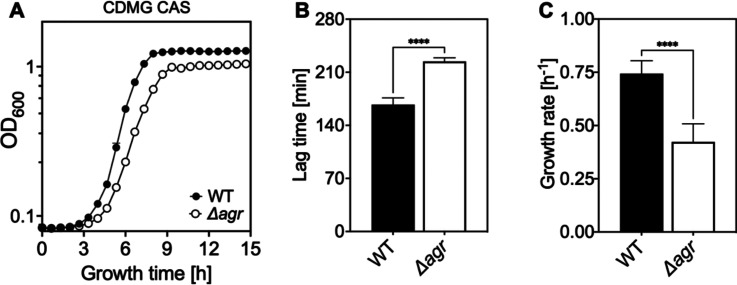
Figure 1—figure supplement 3. Extended lag phase and decreased growth rate and yield of an Δagr mutant.
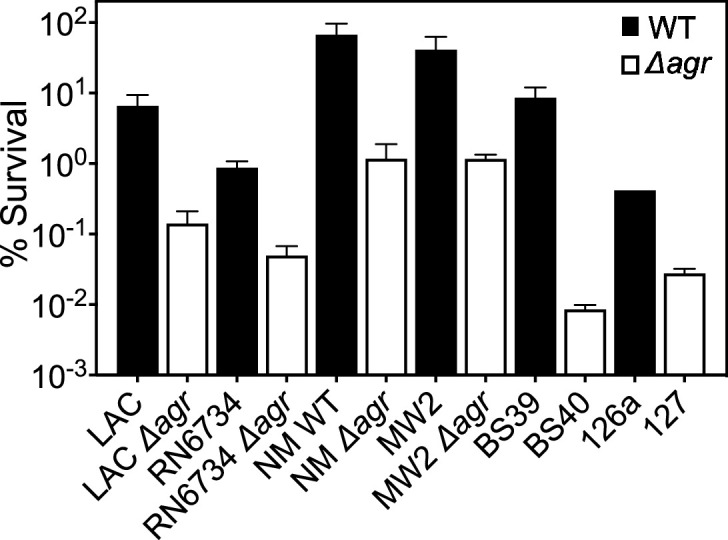
Figure 1—figure supplement 4. Agr-mediated protection from H2O2-mediated killing among diverse S. aureus strains.