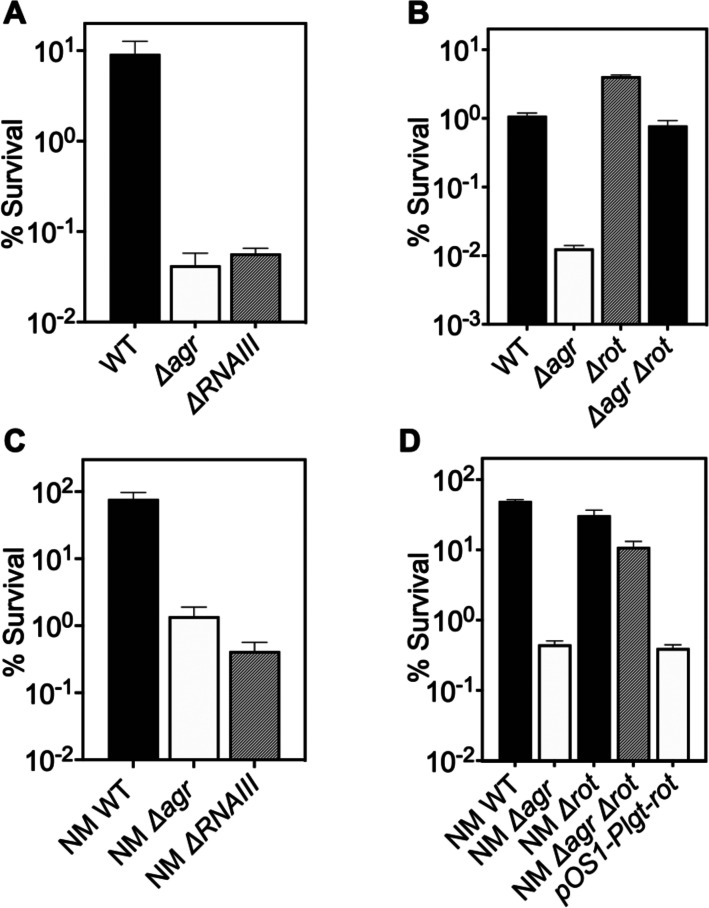
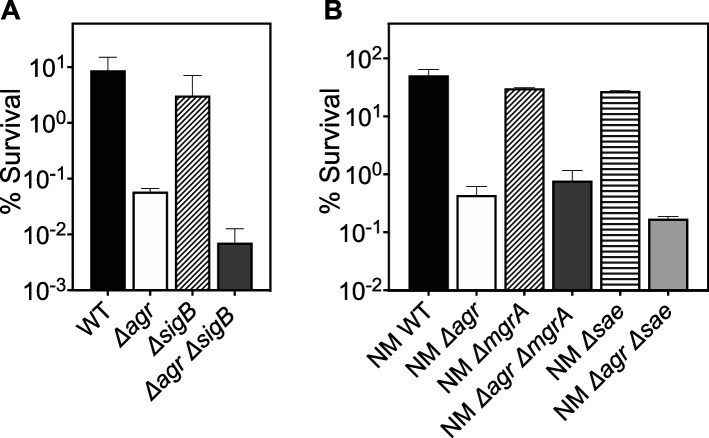
Figure 2. Involvement of RNAIII and rot-dependent pathways in agr-mediated protection from H2O2-mediated killing.
Cultures were grown for 1 hr following dilution from overnight cultures to early log phase (OD600∼0.15) and then treated with 20 mM H2O2 for 60 min before determination of percent survival by plating and enumeration of colonies. (A) Wild-type LAC (WT, BS819), Δagr mutant (BS1348), and ΔrnaIII mutant (GAW183). (B) Δrot and Δagr Δrot double mutant (BS1302). (C) Wild-type (WT) strain Newman (NM, BS12), Δagr mutant (BS13), and ΔRNAIII mutant (BS669). (D) Overexpression of rot. Rot was expressed from a plasmid-borne wild-type rot (pOS1-Plgt-rot, strain VJT14.28). Data represent the mean ± SD. from biological replicates (n=3).