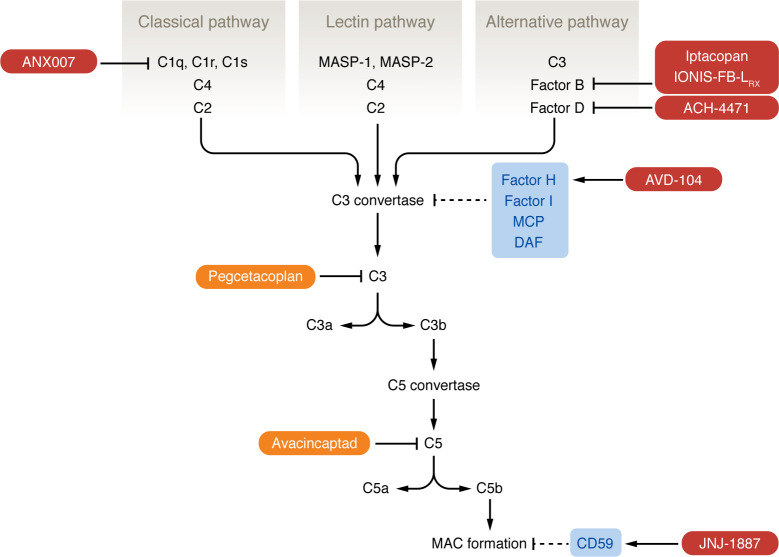
Figure 1. Pathways of the complement system.
The complement system is composed of three pathways (classical, lectin, and alternative) that converge on the formation of a C3 convertase complex that is unique to each pathway. The classical pathway begins with binding of the C1 complex (composed of C1q, C1r, and C1s) to an antigen-antibody complex of pathogen surface directly; this leads to cleavage of C4 and then C2 to form the C3 convertase of the classical pathway. The lectin pathway is similar in that it begins with MBL recognizing mannose residues on a pathogen surface; this activates the MBL-associated serine proteases MASP-1 and MASP-2, which cleave C4 and C2. The alternative pathway is initiated by spontaneous hydrolysis of C3, which binds FB, leading to cleavage of FB by FD; this complex is stabilized by properdin. C3 convertase activity leads to the formation of the C5 convertase and eventually the MAC, triggering membrane destabilization of foreign material. Host complement inhibitors (light blue: FH, FI, MCP, DAF, CD59) target C3 convertase and MAC formation. FDA-approved complement inhibitors for GA (orange) are pegcetacoplan, which targets C3, and avacincaptad, which targets C5. Investigational therapies for GA (dark red) target components of the classical and alternative pathways.