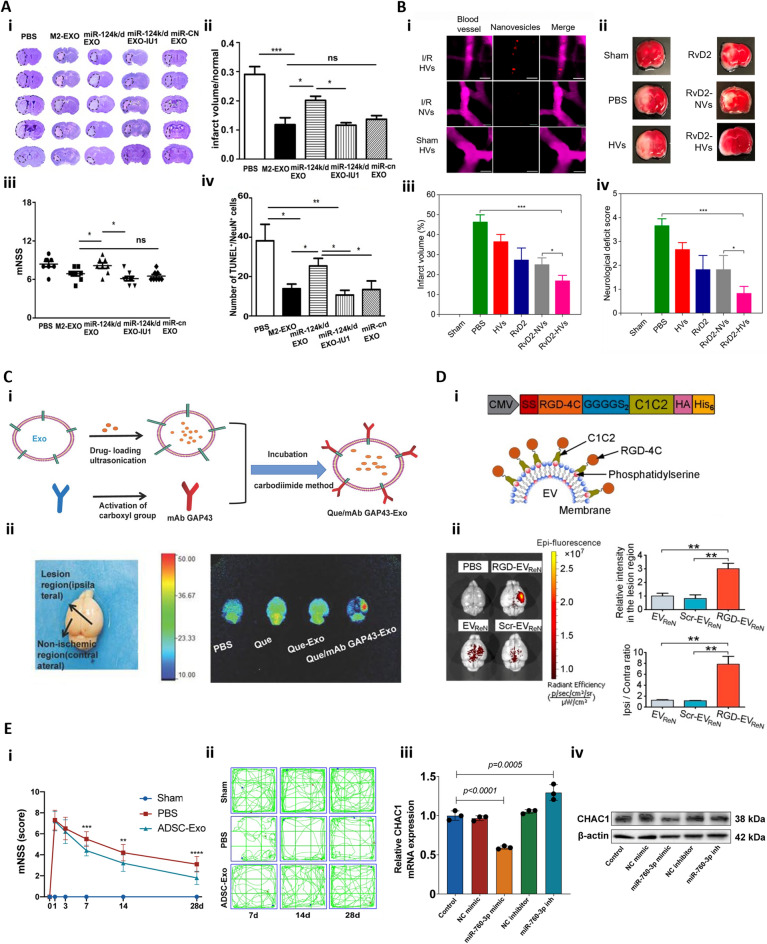
Fig. 2.
Exosome-based therapy for cerebral IRI. A Tail vein injection of M2 microglia-derived exosomes (M2-EXO) attenuated cerebral IRI by downregulating of protease 14 (USP14) expression. (i) Cresyl violet-staining of brain sections, (ii) normalized infarct volume %, (iii) modified neurological severity score (mNSS), and (iv) number of apoptotic neurons in the brain of the mice treated with PBS, M2-EXO, miR-124 k/d EXO, miR-124 k/d EXO + IU1 and miR-cn EXO (exosomes from M2 microglia treated with a control lentivirus vector) [73]. B Intravenous injection of RvD2-loaded artificial exosomes, synthesized from the membranes of differentiated human promyelocytic leukemia cells (HL-60), reduced neurological damage following tMCAO. (i) Confocal images showed Dil-labeled nanovesicles (red), from differentiated HL-60 (HVs), adhering to the inflamed brain vasculature post tMCAO while nanovesicles from non-differentiated HL-60 (NVs) showing minimal binding. Blood vessels visualized by BSA-Cy5 (pink). Scale bar = 20 μm. (ii) TTC-stained brain sections revealed (iii) Infarct sizes and (iv) neurological deficit scores at 22 h after injection Reprinted with permission from [79]. Copyright 2019 American Chemical Society. C Intravenous injection of mAb GAP43-conjugated exosomes targeted specifically to damage neurons post cerebral IRI. (i) Scheme of conjugating mAb GAP43 to quercetin (Que)-loaded exosome surface to assemble Que/mAb GAP43-Exo. (ii) Fluorescence images of brains from tMCAO rats treated with PBS, Que, Que-Exo, and Que/mAb [81]. D Neural progenitor cell-derived exosomes modified with RGD peptides enhanced lesion targeting through intravenous injection. (i) Design of RGD-C1C2 and RGD-C1C2-decorated EV. (ii) Near Infrared Fluorescence images of mice brains the intravenous administration of PBS, Cy5.5-labeled EV, Scr-EV or RGD-EV. Quantitation of fluorescence intensity in the lesion region and ratios of fluorescence intensity in ipsilateral versus contralateral region [76]. E Intranasal administration of exosomes derived from adipose-derived MSC (ADSC-Exo) improved neurobehavior function and inhibited ferroptosis by downregulating CHAC1 expression via miR-760-3p. (i) Neurological deficits were evaluated using mNSS before and after tMCAO. (ii) Motor function was assessed. (iii) RT-qPCR results of CHAC1 mRNA expression. (iv) Western blot showing CHAC1 protein expression [82]