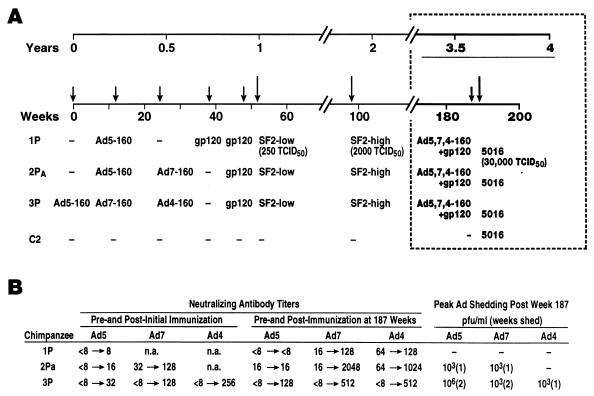
FIG. 2.
Immunization and challenge history of the three previously protected chimpanzees. (A) Prior administrations of one, two, or three HIV-1MN gp160 recombinants based in Ad4, Ad5, or Ad7 vectors and one or two boosts with CHO-expressed gp120 of the HIV-1SF2 strain are listed. The animals were protected against low- and high-dose HIV-1SF2 challenges at weeks 52 and 98, respectively, with no intervening immunizations (24). The timing of the boost and challenge described in this report is outlined in the boxed area. (B) Ad neutralizing antibody titers following initial and booster immunizations are listed. Postimmunization peak titers are listed. Duration of Ad shedding in nasal secretions, expressed in weeks, and peak viral titers of positive samples, expressed as PFU per milliliter, following the booster immunization at week 187 are also listed. Ad titers were adjusted for an ∼10-fold dilution of nasal secretions obtained by swabbing. n.a., not applicable.