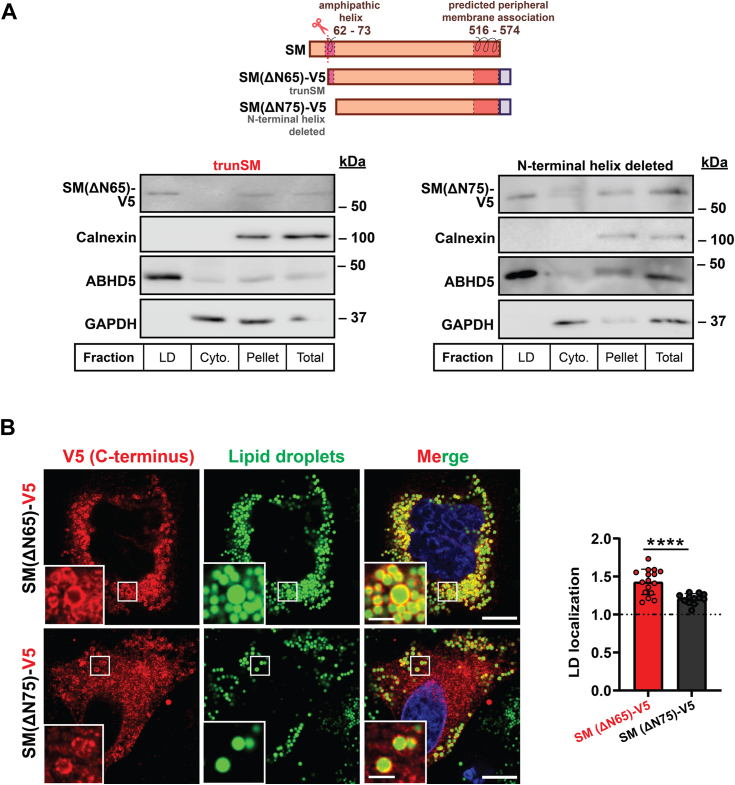
Figure 6.
Deletion of the N-terminal amphipathic helix diminishes trunSM-lipid droplet association.A, HeLa cells were transfected with the indicated constructs for 24 h and treated with 450 μM oleic acid for 24 h. Lipid droplet (LD), cytosol (cyto.), pellet, and total fractions were collected, and levels of calnexin (endoplasmic reticulum marker), ABHD5 (LD marker), GAPDH (cytosol marker), and V5-tagged proteins of interest were determined by immunoblotting. B, confocal microscopy images alongside quantification. Cells were fixed, LDs were stained with BODIPY 493/503, and anti-V5 immunofluorescence was performed. Scale bar indicates 10 μm for main images, and 2 μm for insets. Images are representative. Data are expressed as the mean intensity of V5 staining on LD surface relative to the area surrounding LDs. Values >1 indicate protein–LD surface localization. LD localization is further defined in Experimental procedures and Fig. S2. Data presented as mean ± SD from n = 11 to 16 cells (∗∗∗∗p < 0.0001; ordinary one-way ANOVA). Images were collected across two independent experiments for each construct. ABHD5, abhydrolase domain-containing-5; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SM, squalene monooxygenase; trunSM, truncated SM.