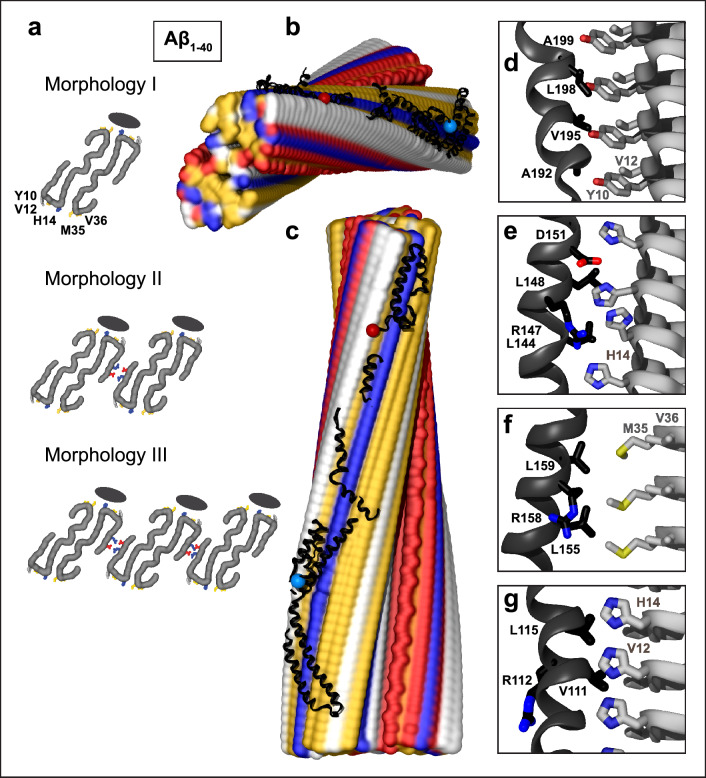
Fig. 1.
Structural model of apoE docked onto Aβ1–40 fibril from AD vasculature. The model was obtained using apoE segments shown in Supplemental Fig. 2d–h and the fibril structure (PDB ID: 6SHS). Segment positions are compatible with full-length apoE. a Fibril morphologies I–III containing one to three paired protofilaments (main chain in gray, view down the fibril axis). Morphology I was used for docking. Black ovals mark the apoE docking position at one of two predicted sites per paired protofilament. ApoE-coordinating residues Y10, V12, H14 from molecule 1 and M35, V36 from molecule 2 of Aβ1–40 protofilament are indicated. In morphologies II and III, residue pairs E3 and R5 that form salt bridges between adjacent protofilaments [64] are shown. b, c Top and side views of the docking model show apoE alongside Aβ1–40 protofilament. In this and other figures of apolipoprotein–amyloid complexes, apolipoprotein main chains are in black ribbons; blue and red dots mark N- and C-termini. Amyloid fibrils are in a surface representation: yellow—hydrophobic, white—polar, red—acidic, blue—basic (including His). Panels d–g show apoE–amyloid contacts within ~ 5 Å in selected regions (apoE—black, Aβ—gray). d Aβ1–40 residue ladder Y10, V12 forms hydrophobic interactions with apoE residues; A192, V195, L198, A199 from the apoE hinge region are shown. e H14 of Aβ1–40 forms mixed (polar/hydrophobic) interactions with apoE residues; R147, L144, L148, D151 from helix 4 are shown. f R158 in apoE3/E4, which flanks the hydrophobic face of helix 4, interacts unfavorably with M35 of Aβ1-40. g Hydrophobic residues of apoE helix 3 interact with residue ladders of V12 and H14 in Aβ1-40. Residue 112 (R112 in apoE4) points away from amyloid