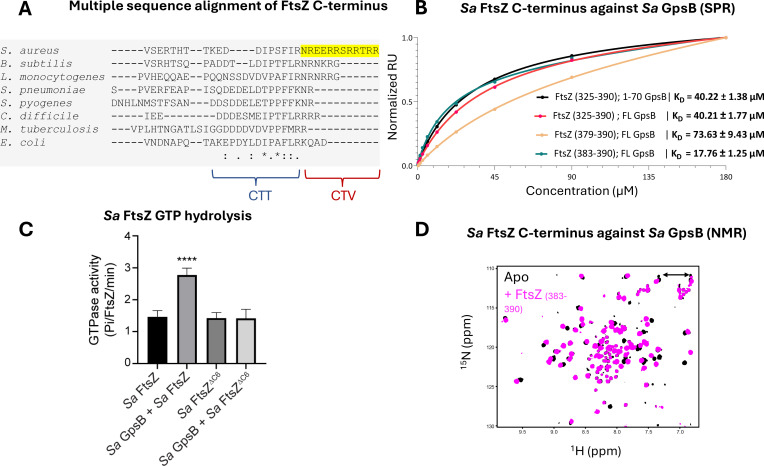
Figure 3. Sa FtsZ contains a repeated GpsB recognition motif at its C-terminus.
(A) A multiple sequence alignment of the FtsZ C-terminus from different representative bacteria reveals that there is a repeated GpsB recognition motif in Sa FtsZ (highlighted region) and that it is unique to this bacterium. (B) Surface plasmon resonance (SPR) titration of peptides corresponding to several segments of Sa FtsZ against Sa GpsB (residues 1–70 or full length). A titration of Sa FtsZ (residues 325–390) against 1–70 GpsB (black), corresponding to the N-terminal domain, shows binding can be isolated to this region. (C) When incubated with Sa GpsB, a Sa FtsZ mutant with a C-terminal truncation (SRRTRR, FtsZΔC6) has significantly lower GTP hydrolysis compared to its full-length counterpart. GTP hydrolysis was measured by monitoring inorganic phosphate (Pi) released (µmoles/min) by either FtsZ or FtsZΔC6 (30 μM) in the absence and presence of GpsB (10 μM). The plot is the average of n=6 independent data sets. p-Value for **** is <0.0001. (D) Overlays of the 1H-15N HSQC of spectrum of Sa GpsB (1–70) in the absence (black) and in the presence of FtsZ (383–390; pink). The boxed region highlights the only sidechain pair of signals that becomes affected by the addition of Ftsz. Based on a model derived from the structure of Bs GpsB in complex with a penicillin-binding protein (PBP)-derived peptide (Figure 1—figure supplement 2), it is tentatively assigned to Q43 of Sa GpsB.