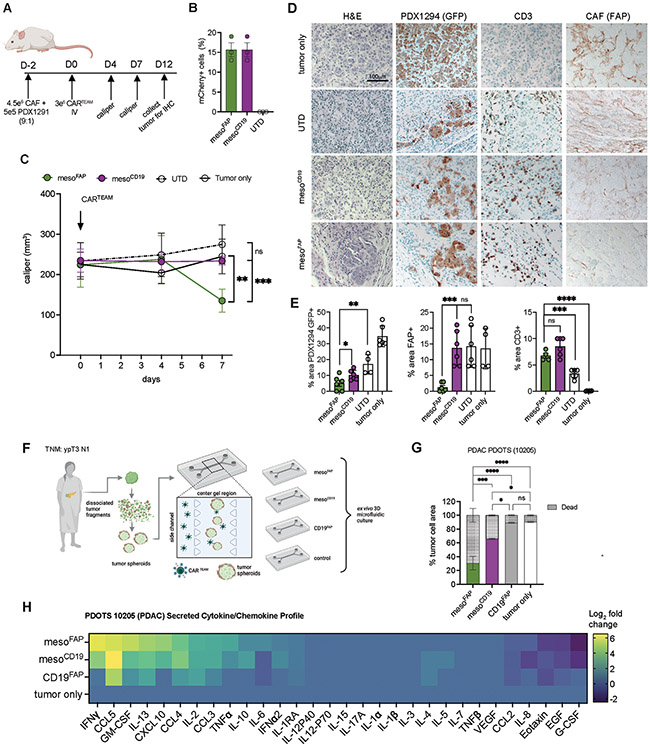
Figure 6. MesoFAP CAR T-cells outperform control CAR T-cells in PDX in vivo and ex vivo models.
A. Schematic of experimental design in which CAFs and PDX1291 cell line (9:1 ratio) were subcutaneously implanted in the flank of NSG mice. Mice were treated intravenously on day 2 post-tumor-CAF implantation with UTD, mesoFAP, and mesoCD19 CAR T-cells. B. Transduction efficiencies of normalized CARTEAM including UTD of in vivo experiment from 3 independent experiments. C. Tumor volume over time as measured by caliper (n=5 mice per group). D. Representative hematoxylin and eosin (H&E) staining and immunohistochemical (IHC) staining for GFP (PDX1294), FAP (CAF), and CD3 (T-cells) on tumors harvested on day 14. Scale bar indicates 100mm. E. Quantification of D. as percent area positive for each marker; 4-6 representative examples quantified. F. Schematic of PDOTS experimental setup including the 3D microfluidic co-culture system with a side channel (where CAR TEAM are added with an E:T of 1:3) and center/gel region (where tumor spheroids are grown in collagen hydrogels). G. Evaluation of live/dead cells on day 5 of mesoFAP-, mesoCD19-, and CD19FAP-treated or tumor-only PDOTS (n=3). H. Secreted cytokine and chemokines analysis as Log2 fold change (relative to control samples) on day 5 of mesoFAP, mesoCD19, CD19FAP, and tumor-only PDOTS from F. Data represents mean +/−SEM. Stars indicate significance as determined by unpaired two-tailed t-tests (panels B and D) or one-way ANOVA with Tukey’s multiple comparisons test (panel F). *p < 0.05, **p < 0.01 ***p < 0.001****p < 0.0001, ns: not significant. Schematics were created with BioRender.com.