Figure 1.
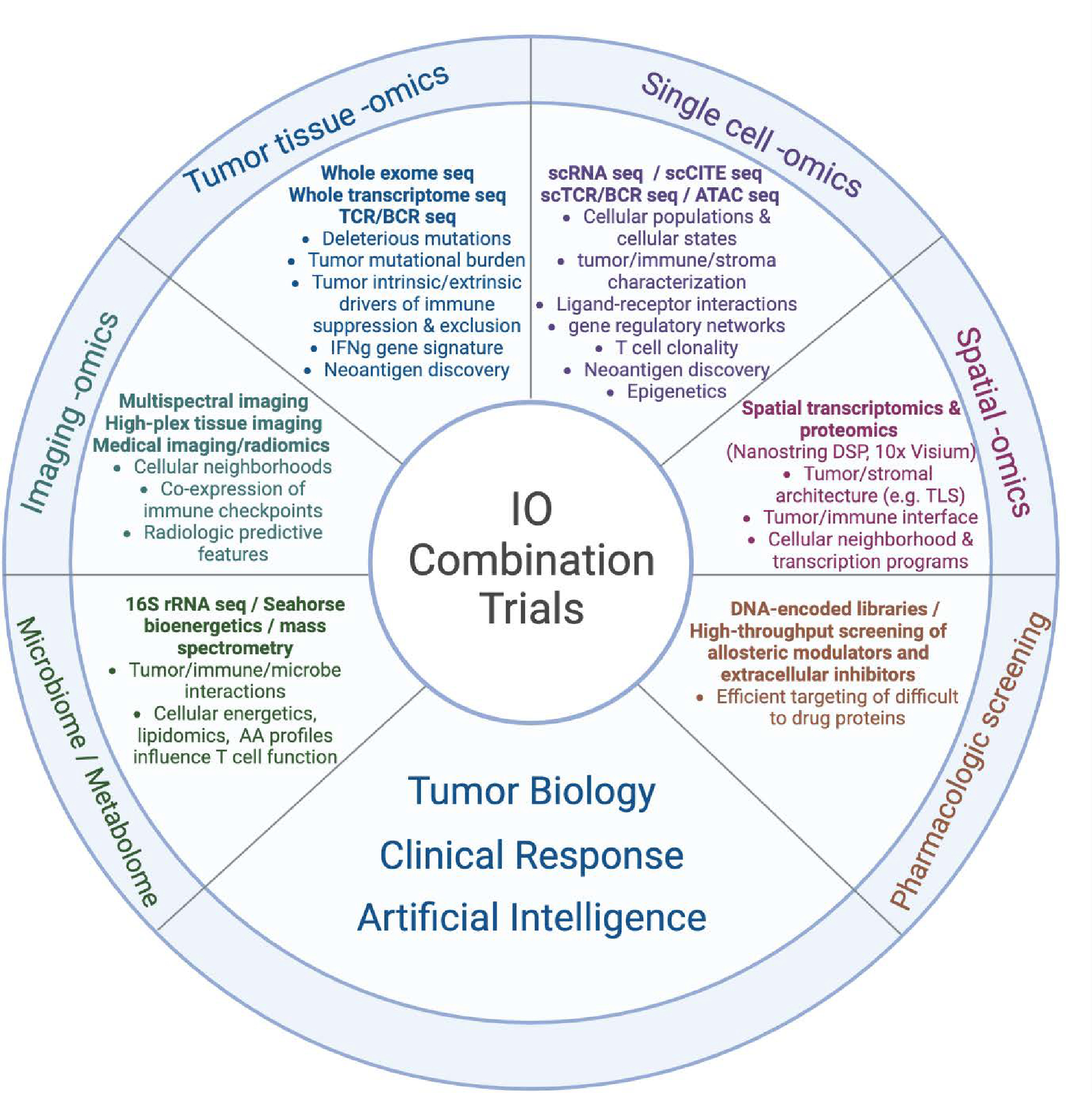
An array of multi-omics technology and AI platforms can be used to characterize the tumor-immune-stromal interface, identify resistance pathways, predict clinical response, and catalyze a new era of combined immunotherapy strategies.
Tumor tissue omics: Includes high-throughput sequencing of the tumor genome, exome, and transcriptome. Specific mutations in the tumor DNA can be detected through whole-genome sequencing (WGS), WES, targeted sequencing (e.g., via companion diagnostic (CDx)), among others. RNAseq of the tumor transcriptome can detect differentially expressed genes in clinically relevant samples that may correspond to targetable pathways. The expression of IFNγ based gene signatures can also be used to categorize tumor samples into T cell-inflamed versus non-T cell-inflamed phenotypes and identify the upregulation of pathways specific to immune exclusion or ICI resistance. Sequencing of the T cell receptor (TCR) or B cell receptor (BCR) may indirectly identify tumor-specific T cell or B cell clonal populations. Neoantigens can be predicted from integration of DNAseq (mutations), RNAseq (expression), and potentially mass spectrometry-based proteogenomics.
Single cell omics: High-throughput profiling of individually isolated cells provides transcript sequence and abundance from specific cell types, providing a high resolution of the tumor microenvironment. Advances in multiplexing technologies can further quantify cell-surface protein expression (CITEseq), TCR/BCR repertoire, and chromatin accessibility (ATACseq) in addition to single cell transcriptomics. By increasing the resolution of gene and protein expression to the cellular level, differentially expressed pathways can be specifically identified within the tumor, immune, and stromal cell populations to further characterize molecular mediators of immune exclusion in the TME. Further, cell surface protein expression via CITEseq can provide additional phenotypic data to determine cell types. TCR sequencing of infiltrating lymphocytes can identify neoantigen-specific TCRs and allow engineering of neoantigen-specific TCRs in vitro.
Imaging omics: An array of technologies including multiplex immunofluorescence, codetection by indexing (CODEX), iterative indirect immunofluorescence imaging (4i), and imaging mass cytometry (among others) now permit dozens of unique protein markers to be digitally measured from fixed tumor tissue. On the macroscopic level, thousands of features from radiologic body imaging can be computationally extracted to build machine learning models in prediction of clinical outcome.
Spatial omics: As another platform to characterize tumor-immune-stromal interactions, both Nanostring digital spatial profiling (DSP) GeoMx and 10X Visium can be used to map gene expression values to fresh frozen or formalin-fixed paraffin embedded (FFPE) tissue, identifying pathways of immune exclusion at a geographical level along with cellular neighborhoods that may be associated with clinical outcomes. Moreover, single-cell resolution has been achieved by Nanostring CosMx single-molecule imaging (SMI), 10x Xenium, and Vizgen MERSCOPE (Multiplexed Error-Robust Fluorescence in situ Hybridization, MERFISH). These technologies profile several hundred to a few thousand targeted genes within individual cells in a spatial context. This advancement allows for a deeper understanding of cell-to-cell communication and interactions between ligands and receptors in cellular neighborhoods of TME.
Metabolomics and the Microbiome: In addition to gene and protein expression, a number of metabolic platforms have been developed to measure parameters of cellular energetics, including oxygen consumption, extracellular acidification, and lipid and amino acid profiling. Comparing the microbiota of ICI responders versus non-responders using 16s rRNA sequencing, metagenomics, and metatranscriptomics has also identified unique taxa correlated with clinical outcomes.
Pharmacologic screening: While multi-omic platforms can be used to identify novel pathways of IO resistance, advances in pharmacologic screening are required to target these pathways in clinical trials. DNA encoded libraries allow high-throughput screening of millions of compounds to efficiently identify candidate agents to target historically “undruggable” proteins.
Artificial intelligence: Machine learning (ML) and deep learning (DL) have been pivotal in driving our understanding and therapeutic approaches to diseases at a personalized level, including but not limited to, multimodal data integration across radiology scans, pathology images, electronic health records (EHR), and multi-omics; predictive modeling on patient stratification/selection, adverse event monitoring, and immunotherapy treatment response; new immunotherapeutic agent discovery or drug repurposing from in vitro screens and/or cell imaging; and foundation models for medical imaging segmentation, single-cell phenotypic annotation, and drug discovery from chemical language representations. As technology and data evolve, it’s anticipated that AI will play an even more significant role in propelling these fields forward.
IO, immuno-oncology; sc, single-cell; seq, sequencing; CITE, cellular indexing of transcriptomes and epitopes; TCR, T cell receptor; BCR, B cell receptor; ATAC, assay for transpose-accessible chromatin; DSP, digital spatial profiler; TLS, tertiary lymphoid structure; IMC, imaging mass cytometry; CODEX, co-detection by indexing; AA, amino acid
Figure made with BioRender
