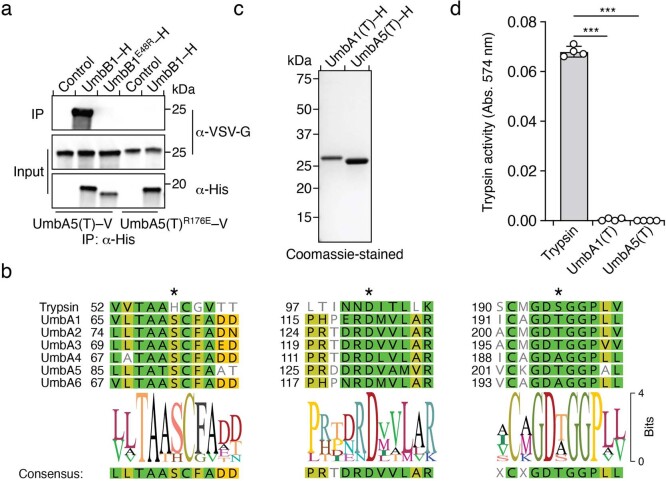
Extended Data Fig. 4. The trypsin-like domain of UmbA proteins mediates binding with UmbB and lacks catalytic activity.
a, WB analysis from IP experiments of the indicated heterologously expressed, tagged Umb proteins. UmB1E48R–H and UmbA5(T)R176E–V contain substitutions of residues predicted to be critical for interaction between the two proteins. Experiment shown is representative of two independent replicates. b, Structure-guided alignments of the UmbA(T) regions normally encompassing the catalytic histidine, aspartate, serine triad typical of trypsin proteins, indicating the conserved substitutions found across the UmbA proteins of S. coelicolor. c, d, Coomassie-stained SDS-PAGE analysis (c) and proteolytic activity (d) of purified, heterologously expressed UmbA1(T) and UmbA5(T). Data in (d) represent mean ± SD (n = 4 technical replicates from one experiment, representative of two biological replicates conducted). Asterisks indicate significant differences from the positive control, porcine trypsin (p < 0.0001, two-tailed t-test).