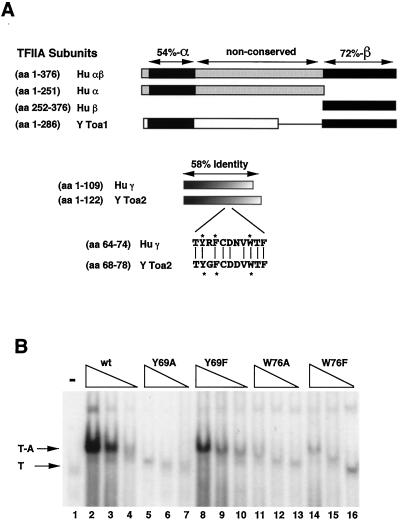
FIG. 1.
TFIIA is a highly conserved heterodimer. (A) The TFIIA subunits from humans (Hu) and yeast (Y) are aligned. The human TFIIA large subunit (αβ) (aa 1 to 376) is aligned to yeast Toa1 (aa 1 to 286). Hu αβ is proteolyzed in vivo to produce individual α and β subunits, which are indicated. The human α subunit is 54% conserved with the yeast homolog (Toa1; aa 7 to 58), while the β subunit is 72% conserved (Toa1; aa 226 to 286). The γ subunit is 58% conserved throughout its length. The conserved Toa2 residues that are mutated in this study are indicated by stars. (B) Yeast TBP-TFIIA complex formation in EMSA. Recombinant yeast TBP and TFIIA proteins were expressed in E. coli, purified, and used in EMSA. The 32-P labeled 30-bp adenoviral E1B TATA box was used as a probe. Decreasing amounts (250, 50, and 20 ng) of TFIIA proteins were added to the EMSA reaction for wt and Toa2 mutant proteins, as indicated above the gel. Arrows point to the TBP-DNA (T) and TBP-TFIIA-DNA (T-A) complexes.