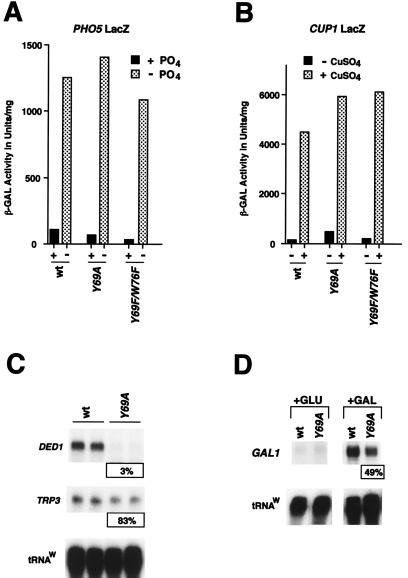
FIG. 5.
A stable T-A complex is not generally required for transcription in vivo. (A) PHO5-driven LacZ expression at 30°C in SC medium was assayed for wt, toa2 Y69A, and Y69F/W76F strains by β-Gal assays. In high-PO4 medium, PHO5 expression was repressed. In synthetic medium in the absence of PO4, PHO5 induction is shown. β-Gal activity is expressed as units per milligram of protein. (B) CUP1-driven β-Gal activity was assayed under conditions similar to those for the experiment for which results are shown in panel A, except that after reaching mid-log phase, samples were grown for an additional 4 h at the nonpermissive temperature (37°C) prior to Cu ion addition. (C) The toa2 Y69A mutant shows defective expression of endogenous DED1, but not of TRP3, in vivo. The wt and toa2 Y69A mutant strains were grown to mid-log phase in SC medium at 30°C and were shifted to the nonpermissive temperature (37°C) for 3 h prior to RNA isolation. Endogenous DED1 and TRP3 expression was assayed by S1 nuclease protection. (D) GAL1 expression is induced in the toa2 Y69A mutant. The wt and toa2 Y69A strains were used to assay GAL1 expression in vivo. Expression levels were measured under conditions of glucose repression (+GLU) and under galactose induction (+GAL). PhosphorImager quantitation indicates that GAL1 expression was induced about 600-fold in the wt and 300-fold in the toa2 Y69A strain. For the GAL1 samples, 4 μg of total RNA was used per reaction, while 40 μg of total RNA was used for all other S1 reactions. PhosphorImager quantitation (expression in the mutant as a percentage of that in the wt) is shown in panels C and D.