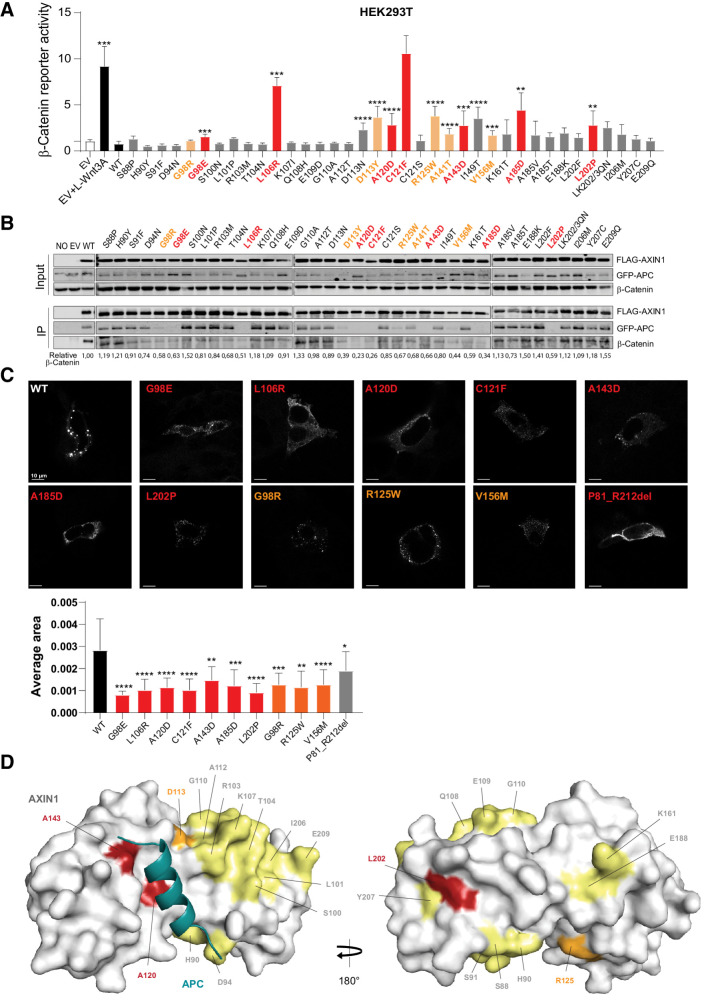
Figure 5.
Analysis of missense variants within the RGS/APC domain. Tumor-associated missense variants in the RGS/APC domain were analyzed for their defect in β-catenin regulation, APC and β-catenin binding, and capacity to induce intracellular puncta formation. A, A β-catenin reporter assay was performed to determine the defect in β-catenin regulation for 37 RGS/APC domain variants. Red-marked bars represent variants that show a near-complete loss of GFP-APC and/or β-catenin binding (see B), whereas orange bars represent variants with partial loss. All β-catenin reporter activities are depicted as WRE/CMV-Renilla ratios (in triplicate, three independent experiments), in which the value obtained for the empty vector (EV) was arbitrarily set to 1. B, Immunoprecipitation assay to determine the binding capacity of missense variants to cotransfected GFP-APC or endogenous β-catenin. C, Immunofluorescence analysis of transfected AXIN1 missense variants to determine their puncta formation capacity. Average dot size was determined using ImageJ software on at least 6 independent cells. Data are shown as mean ± SD. D, Surface representation of AXIN1 (gray) with bound APC helix (deep teal). Amino acid residues mutated in this study that are located on the surface of AXIN1 are labeled and colored according to extent of loss of APC binding (B), with yellow indicating no loss, orange indicating partial loss, and red indicating near-complete loss of APC binding. Statistical significance for all experiments was analyzed using a Mann–Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.