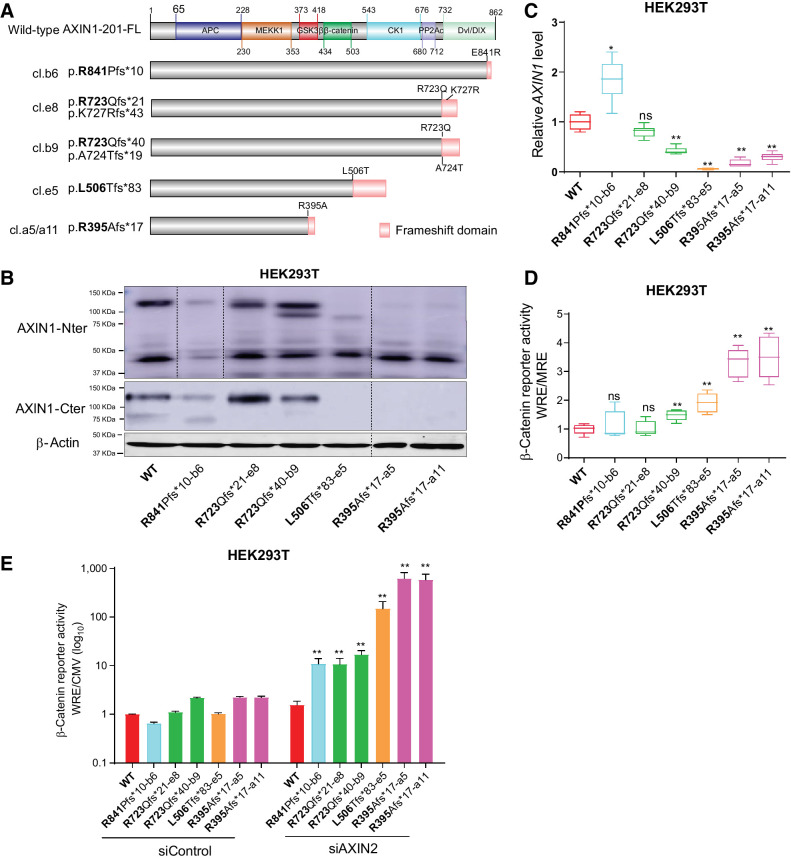
Figure 7.
Characterization of AXIN1-truncating mutations in regulating β-catenin signaling. Analysis of clones carrying various endogenous AXIN1 truncations generated in HEK293T cells using CRISPR/Cas9-mediated gene editing. A, Schematic diagram showing the AXIN1 truncation variants generated in HEK293T cells using gene editing. The red domain at the end of truncated variants represents amino acid stretches resulting from the introduced frameshift mutation. B, Immunoblot analysis of generated clones using AXIN1 antibodies with N- and more C-terminal epitopes (cat. # 3323S and cat. #2087S, Cell Signaling Technology). Images were generated from one blot for each antibody, with irrelevant lanes removed. Original blots can be seen in Supplementary Data S1. C, qRT-PCR assay to determine AXIN1 mRNA expression levels (in triplicate, two independent experiments). Expression levels are depicted relative to the housekeeping gene GAPDH. The value for the WT control was arbitrarily set to 1. D, A β-catenin reporter assay was performed to determine β-catenin signaling levels in AXIN1-truncated clones. Values are depicted as WRE/MRE ratios (mean ± SD; in triplicate; three independent experiments). The value for the WT control was arbitrarily set to 1. E, Following siRNA-mediated knockdown of AXIN2, a β-catenin reporter assay was performed in all AXIN1-truncated clones. Values are depicted relative to the WRE/CMV-Renilla ratios obtained for the siControl-WT, which was arbitrarily set to 1. Note the logarithmic scale. Data are shown as mean ± SD. Statistical significance for all experiments was analyzed using a Mann–Whitney test. *, P < 0.05; **, P < 0.01; ns, nonsignificant.