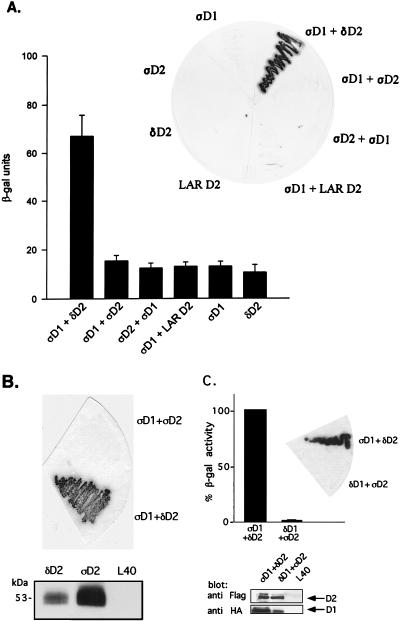
FIG. 3.
Binding of PTPς-D1 to PTPδ-D2, but not of PTPδ-D1 to PTPς-D2, analyzed by yeast two-hybrid binding assays. (A) Quantitative liquid β-gal assays (histograms) or filter β-gal assays (inset) performed with the bait PTPς-D1 (ςD1) fused to the LexA DNA binding domain and the prey PTPδ-D2 (δD2), PTPς-D2 (ςD2), or LAR D2 fused to the Gal4 transactivation domain. Interactions between D1 and D2 of PTPς were also analyzed in the opposite configuration (LexA-ςD2 and Gal4-ςD1, third bar from the left and third yeast streak clockwise from the top in the inset). The quantitative β-gal assays represent means ± standard errors of six determinations. (B) Protein expression of FLAG-tagged D2 domains of PTPς (ςD2) and PTPδ (δD2) in the pACT vector, demonstrating strong expression of both proteins in yeast L40 cells (bottom) but an association of ςD1 with δD2 only and not with ςD2 upon coexpression in L40 cells (top). (C) Lack of binding of PTPδ-D1 to PTPς-D2. Filter β-gal assays (top, inset) or liquid β-gal (top) assays were performed with the bait LexA–PTPδ-D1 and the prey Gal4–PTPς-D2 cotransformed into yeast L40 cells or with the positive control LexA–PTPς-D1 plus Gal4–PTPδ-D2. Bars represent means ± standard errors (n = 8). The actual β-gal activities were 445.7 ± 27.6 U for ςD1 plus δD2 and 6.4 ± 1.2 U for δD1 plus ςD2. For analysis of protein expression, L40 cells untransformed or transformed with δD1 plus ςD2 or with ςD1 plus δD2 were lysed and proteins were separated by SDS–10% PAGE and immunoblotted with anti-FLAG (against D2) or anti-HA (against D1) antibodies (bottom).