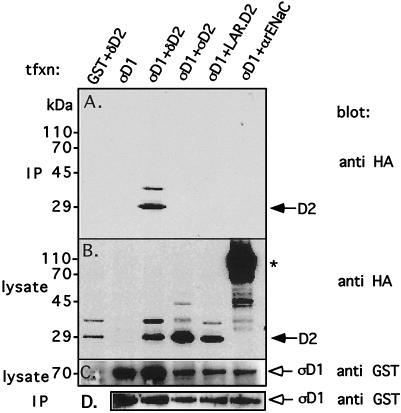
FIG. 4.
Coprecipitation of PTPς-D1 and PTPδ-D2 in mammalian cells. PTPς-D1 expressed as a GST fusion protein (in the mammalian expression vector pEBG) was transiently cotransfected with either HA-tagged PTPδ-D2, PTPς-D2, or LAR-D2 (in pCMV4) into Cos7 cells. Transfected cells were lysed, the lysate was incubated with glutathione agarose beads to precipitate GST–PTPς-D1 and associated proteins, and the proteins were separated on by SDS–10% PAGE and immunoblotted with anti-HA antibodies to detect coprecipitated HA-tagged D2 domains (panel A, IP). Aliquots of the lysate were also analyzed for levels of expression of either the HA-tagged D2 domains by using anti-HA antibodies (panel B, lysate) or GST–PTPς-D1 by using anti-GST antibodies (panel C, lysate). The blot in panel A was then stripped and reprobed with anti-GST antibodies to determine the amount of GST–PTPς-D1 precipitated from the cell lysates (panel D, IP). The reason for the appearance of a slower-migrating band (∼35 kDa, recognized by both anti-HA and anti-D2 antibodies) in lanes representing the D2 domains of PTPς, PTPδ, and LAR (B) is not known, but these bands do not represent phosphorylated forms of the proteins (data not shown). The asterisk marks the HA-tagged α subunit of the rat epithelial Na+ channel (αrENaC), which was used as a negative control for these experiments. tfxn, transfection.