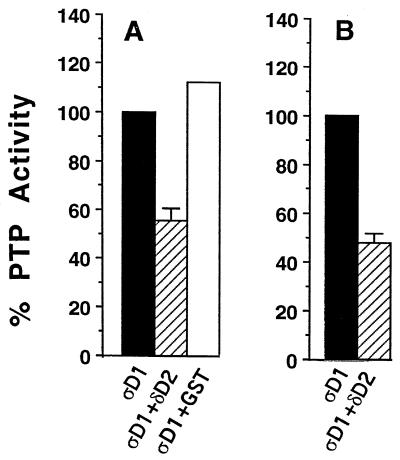
FIG. 5.
Inhibition of catalytic activity of PTPς-D1 by PTPδ-D2. (A) Bacterially expressed GST fusion protein of PTPς-D1 (ςD1), either soluble or immobilized on glutathione agarose beads, was incubated with 100 mM PNPP either alone, with 500 ng of bacterially expressed soluble PTPδ-D2 (δD2), or with GST (control). The reaction was stopped with 0.9 ml of 1 M NaOH, and the optical density at 450 nm of the p-nitrophenolate product was measured. These data are means ± standard errors of four independent experiments. GST–PTPδ-D2 alone had no catalytic activity (data not shown). (B) Inhibition of the catalytic activity of PTPς-D1 (ςD1) by PTPδ-D2 (δD2) coprecipitated from Cos7 cells. The activity of ςD1 precipitated from Cos7 cells, transfected with either ςD1 alone or cotransfected with ςD1 plus δD2, was analyzed as described for panel A. The data are the means ± standard errors of nine independent experiments.