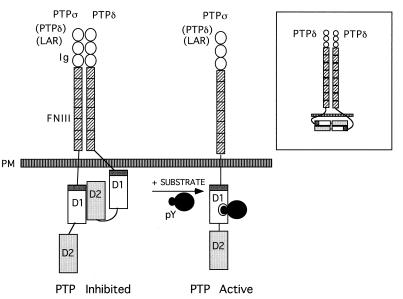
FIG. 8.
Model of D1-D2 heterodimerization of LAR family PTPs. Under resting conditions, the first catalytic domain (D1) of PTPς (or possibly other LAR family PTPs [in parentheses]) is associated with the second catalytic domain (D2) of PTPδ, an association requiring the wedge sequence of PTPς (dark grey). Such intermolecular dimerization inhibits catalytic activity, thus keeping the phosphatase in a partially inactive state. Our current data do not support the association of PTPς-D1 with PTPς-D2 or LAR-D1 with LAR-D2 but cannot preclude the possibility of a weak inter- or intramolecular interaction of PTPδ-D1 with PTPδ-D2 (inset). We speculate that upon presentation of the as-yet-unidentified tyrosine-phosphorylated substrate (likely of high affinity), the D1-D2 heterocomplex is likely to dissociate, thus allowing substrate dephosphorylation. PM, plasma membrane.