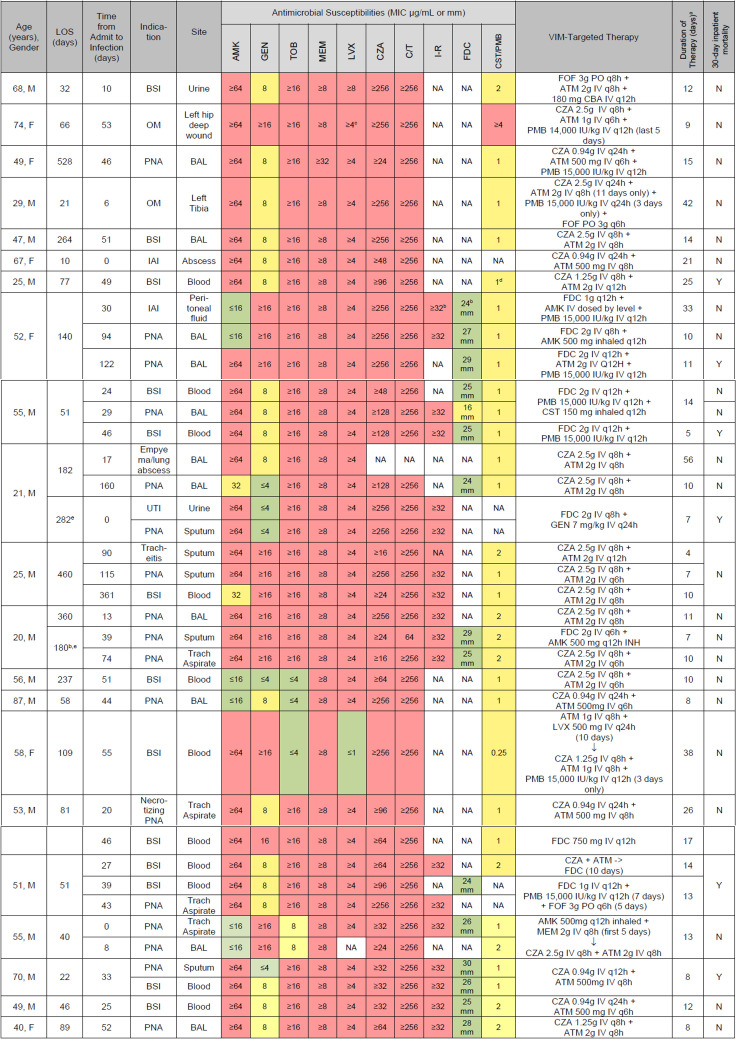
Fig 3.
Isolate susceptibilities and targeted VIM therapies. Includes all infectious episodes treated per patient; only the first isolate per infection. For bacteremia cases, the source isolate also listed when applicable. All doses adjusted for renal impairment by our ASP pharmacists. Abbreviations: BAL, bronchoalveolar lavage; DDKT, deceased donor kidney transplant; EOT, end of therapy; ET, endotracheal; inh, inhaled; IU, international units; IV, intravenous; MIC, minimum inhibitory concentration; OM, osteomyelitis; PNA, pneumonia; PO, by mouth drugs; AMK, amikacin; GEN, gentamicin; TOB, tobramycin; MEM, meropenem; LVX, levofloxacin; FOF = fosfomycin. aTotal duration of ATM plus CZA, or FDC backbone; alternative agents used empirically or as adjunction therapy are listed with their total durations in parenthesis. bCefiderocol and imipenem–cilastatin–relebactam approved and first available at our institution. cLevofloxacin breakpoint changed for Pseudomonas. dCLSI breakpoints for polymyxin B changed for Pseudomonas beginning Jan 2020: <2, intermediate; >4, resistant; no susceptible breakpoint. eSecond hospitalization during the study period.