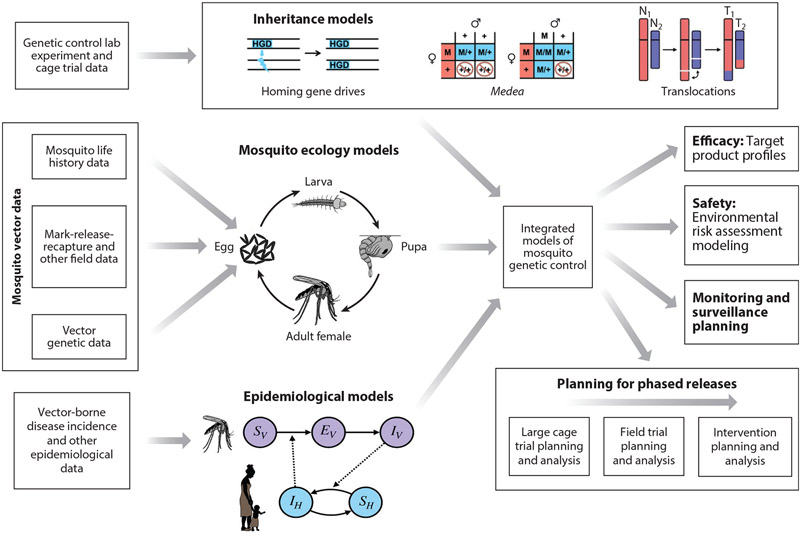
Figure 3.
Integrated models of mosquito genetic control. CRISPR-based genetic control tools are mainly applied to mosquito disease vectors, particularly malaria vectors, due to the huge global health burden of vector-borne diseases, and the potentially transformative role genetic control tools could play in their suppression. As genetic control projects progress from lab to field, models may be used to combine data from disparate sources, including lab, field, and epidemiological studies. These data inform models describing inheritance, population dynamics, and disease transmission, which may be combined into an integrated modeling framework. This integrated framework, or components thereof, may address a range of project-related modeling questions regarding product safety and efficacy and the planning and analysis of cage trials, field trials, and interventions. Pictured here are schematics for (a) homing gene drives (HGDs), which bias inheritance in their favor by converting a heterozygote into a homozygote via cleaving the wild-type allele and then serving as a template for homology-directed repair; (b) Medea, which biases inheritance in its favor through the action of a maternal toxin and zygotic antidote, rendering wild-type (+/+) offspring of heterozygous (M/+) females unviable; and (c) chromosomal translocations (T1 and T2), which result from mutual exchange between terminal segments of two nonhomologous chromosomes (N1 and N2) that provides a selective disadvantage for translocation heterozygotes and threshold-dependent dynamics. Also depicted are (a) the lumped age-class model of mosquito population dynamics, in which the egg, larval, pupal, and adult life stages are modeled, with density-dependence often incorporated at the larval stage and (b) the Ross–Macdonald model, which is the simplest model of reciprocal transmission of malaria between humans and mosquitoes. In the Ross–Macdonald model, adult female mosquitoes emerge susceptible (SV), become exposed/latently infected (EV) at a rate proportional to the number of infectious humans in the population (IH), and progress to infectiousness (IV) at a rate determined by the extrinsic incubation period. Susceptible humans (SH) become infected/infectious at a rate proportional to IV and recover to become susceptible again.