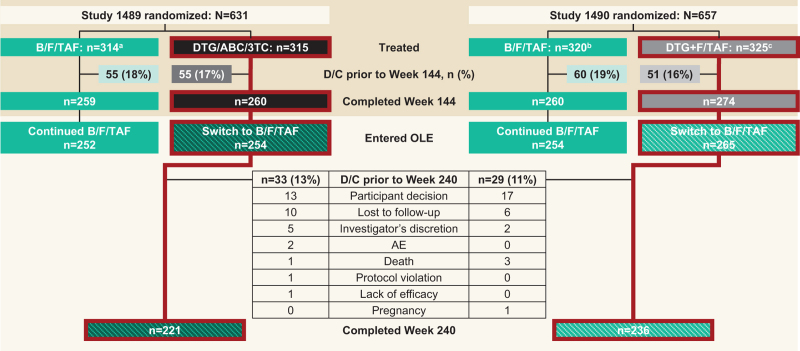
Fig. 1.
Participant disposition from baseline to Week 240 (OLE Week 96).
aTwo participants randomized and not treated; bSeven participants randomized and not treated; cFive participants randomized and not treated. AE, adverse event; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; D/C, discontinuation; DTG/ABC/3TC, dolutegravir/abacavir/lamivudine; DTG+F/TAF, dolutegravir plus emtricitabine and tenofovir alafenamide; OLE, open-label extension.