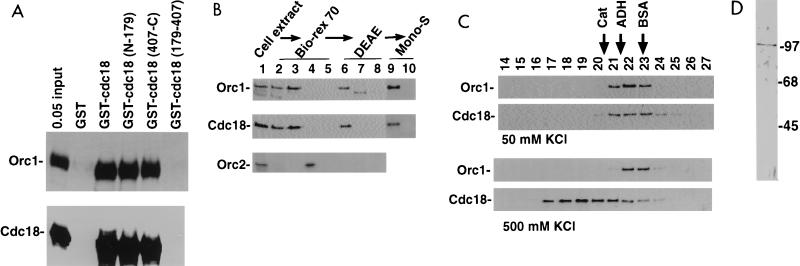
FIG. 4.
Association of Cdc18 protein with Orc1 protein. (A) Glutathione-agarose beads coated with GST, GST-Cdc18 protein, or indicated deletion derivatives prepared in Escherichia coli were incubated with Orc1 protein or Cdc18 protein produced by in vitro transcription-translation in rabbit reticulocyte lysates. Bound proteins were visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorography. (B) Immunoblots with anti-Orc1 protein, anti-Cdc18 protein, and anti-Orc2 protein antibodies. Lane 1, HeLa cell extract was fractionated over a Bio-Rex 70 column; lane 2, flowthrough of 0.15 M K acetate; lane 3, 0.3 M K acetate eluate containing 7% of input protein; lane 4, 0.6 M K acetate eluate; lane 5, 1.5 M K acetate eluate. The 0.3 M eluate was fractionated on DEAE-Sepharose. Lane 6, flowthrough at 0.2 M K acetate containing 57% of the input protein; lane 7, 0.35 M K acetate eluate; lane 8, 1 M K acetate eluate wash. The flowthrough in lane 6 was passed over a Mono-S column. Lane 9, flowthrough at 0.2 M KCl containing 25% of the input protein; lane 10, eluate at 0.6 M KCl. (C) Orc1 and Cdc18 proteins coelute as a heterodimer of 150 kDa upon gel filtration at 50 mM KCl (top) but are separated at 500 mM KCl (bottom). The flowthrough from the Mono-S column was loaded on a Superose 12 column. Fractions were immunoblotted with anti-Orc1 protein and anti-Cdc18 protein antibodies. The positions of elution of the molecular mass markers catalase (Cat; 300 kDa), alcohol dehydrogenase (ADH; 150 kDa), and bovine serum albumin (BSA; 68 kDa) are indicated. (D) Silver stain of the fraction containing Orc1 protein purified to homogeneity. The numbers indicate molecular masses in kilodaltons.