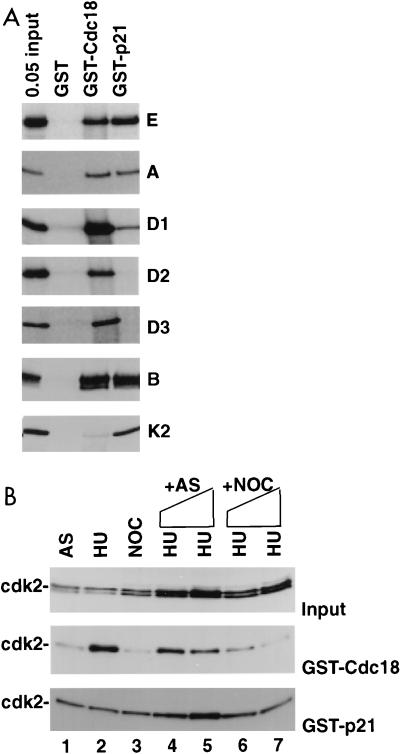
FIG. 5.
Cdc18 protein associates with cyclin-cdk’s through the cyclin, and the association is regulated by a mitotic inhibitor. (A) Indicated S35-labeled proteins were produced by in vitro transcription-translation and tested for association with bacterially produced GST, GST-Cdc18, and GST-p21. 0.05 input, 5% of the input protein incubated with indicated glutathione-agarose beads. Proteins tested were cyclins E, A, D1, D2, D3, and B and cdk2 (K2). (B) Association of cdk2 with GST-Cdc18 is inhibited by a mitotic factor. The immunoblots show input cdk2 (top), cdk2 bound by GST-Cdc18 (middle), and cdk2 bound by GST-p21 (bottom). The extracts are from asynchronous (AS), hydroxyurea-blocked (HU), and nocodazole-blocked (NOC) HeLa cells. Lanes 1 to 3, 0.5-mg extracts directly incubated with glutathione-agarose beads coated with GST fusion proteins produced in bacteria; lanes 4 to 7, the same amount of HU extract used in lane 2 was incubated with increasing amounts of asynchronous cell extracts (lane 4, 0.5 mg; lane 5, 1 mg) or nocodazole-blocked mitotic cell extracts (lane 6, 0.5 mg; lane 7, 1 mg) before incubation with glutathione-agarose beads.