Thromboembolic events frequently occur in patients with COVID-19, due to the underlying hypercoagulable and inflammatory states. However, very few cases of marantic endocarditis have been reported. Herein, we present a rare case of marantic endocarditis with recurrent thromboembolic events in a woman with COVID-19 infection, and in whom an intestinal tumour was found 18 months later. She suffered from cardiogenic shock following 2 embolic inferior ST-elevation myocardial infarctions (STEMIs). She also developed right femoral artery thrombosis and had 2 occurrences of catheter-related venous thrombosis. Marantic endocarditis cases typically are seen with advanced malignancy, but ensuring a thorough medical follow-up can help detect insidious or delayed-onset malignancy.
Marantic endocarditis, also called nonbacterial thrombotic endocarditis (NBTE), is a condition caused by sterile vegetations composed of platelets and fibrin on heart valves. NBTE carries a high risk of valvular dysfunction and systemic embolism. Underlying conditions that are associated with NBTE are advanced malignancy, and hypercoagulable and inflammatory diseases, such as systemic lupus erythematosus, antiphospholipid syndrome, and rheumatoid arthritis.1
Thrombotic and thromboembolic phenomena are found frequently in patients with COVID-19, due to the underlying hypercoagulable and inflammatory states.2 To our knowledge, only 6 cases of NBTE have been reported in the setting of COVID-19.3, 4, 5, 6 However, in 2 of these cases, the patient also had a diagnosis of cancer.3,5 Herein, we present a rare case of marantic endocarditis with recurrent thromboembolism, with COVID-19 disease, and an initially negative cancer screening.
Case Report
A 48-year-old woman presented to the emergency department of a community hospital with chest pain. She had tested positive for severe acute respiratory syndrome (SARS)-CoV-2 11 days prior on a rapid antigen test. She did not have clinical symptoms of COVID infection upon her arrival, and whether she had any symptoms at the time of her initial test is unclear. She had a history of the following: severe IgE-mediated asthma that resulted in a hypoxic cardiac arrest 5 months prior (with evidence of normal coronary arteries on cardiac catheterization); active smoking; hypertension; obesity (body mass index, 43); and obstructive sleep apnea.
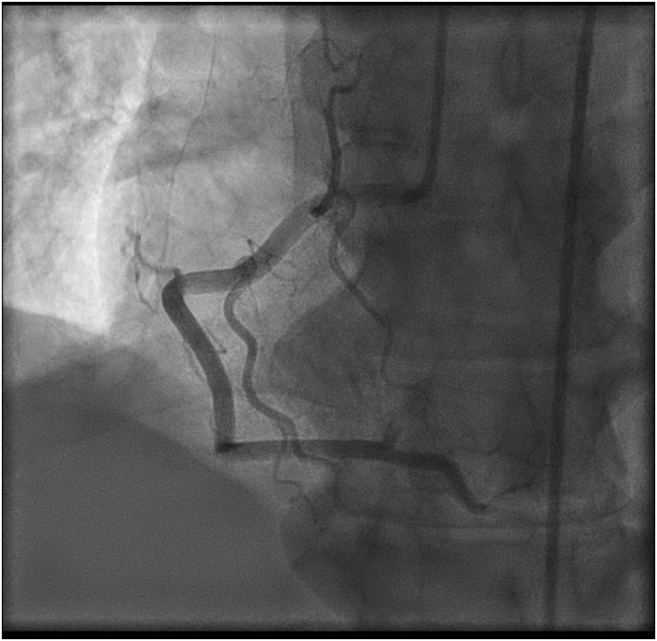
The patient was diagnosed with inferior STEMI. She underwent an urgent coronary angiogram that showed a completely occluded posterior descending artery and posterolateral branches (Fig. 1; Video 1
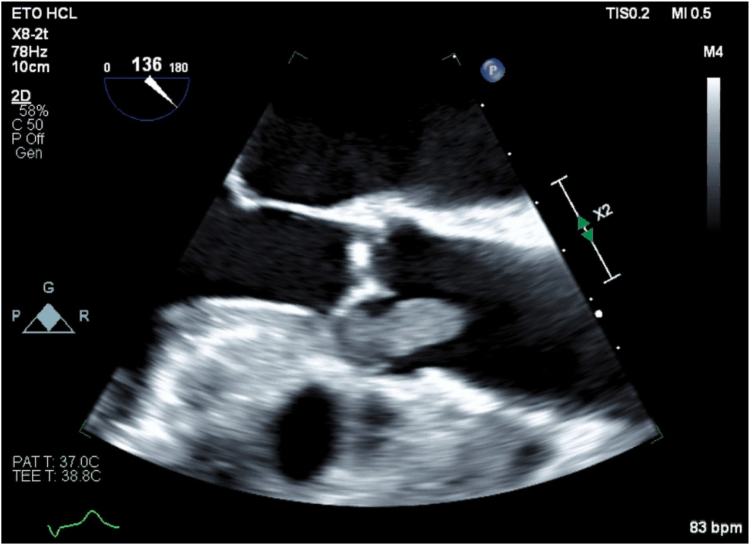
 , view video online). An aspiration thrombectomy was performed, and pathology revealed only white clots. Balloon angioplasty was performed on the posterolateral branches and restored TIMI 2 (thrombolysis in myocardial infarction) flow. No stents were deployed. An embolic origin was suspected for her acute coronary syndrome, given the angiographic appearance of the occlusion. Aspirin and intravenous heparin were initiated. Although it was not seen initially on transthoracic echocardiogram, transesophageal echocardiography (TEE) revealed a large 21 × 9 mm hypermobile mass attached to the right coronary leaflet of the aortic valve (Fig. 2; Videos 2 and 3
, view video online). An aspiration thrombectomy was performed, and pathology revealed only white clots. Balloon angioplasty was performed on the posterolateral branches and restored TIMI 2 (thrombolysis in myocardial infarction) flow. No stents were deployed. An embolic origin was suspected for her acute coronary syndrome, given the angiographic appearance of the occlusion. Aspirin and intravenous heparin were initiated. Although it was not seen initially on transthoracic echocardiogram, transesophageal echocardiography (TEE) revealed a large 21 × 9 mm hypermobile mass attached to the right coronary leaflet of the aortic valve (Fig. 2; Videos 2 and 3
 , view videos online). The aortic valve was otherwise normal, without stenosis or regurgitation. TEE also showed a left ventricular ejection fraction of 55%, with inferior wall motion abnormality and a normal right ventricle. Empiric antibiotic therapy (ceftriaxone, vancomycin, and doxycycline) was initiated for presumed infective native valve endocarditis. A cerebral computed tomography (CT) scan did not reveal cerebral emboli.
, view videos online). The aortic valve was otherwise normal, without stenosis or regurgitation. TEE also showed a left ventricular ejection fraction of 55%, with inferior wall motion abnormality and a normal right ventricle. Empiric antibiotic therapy (ceftriaxone, vancomycin, and doxycycline) was initiated for presumed infective native valve endocarditis. A cerebral computed tomography (CT) scan did not reveal cerebral emboli.
Figure 1.
Right coronary angiogram in left anterior oblique 30 degrees, showing occlusion of the posterior descending artery and posterolateral branches.
Figure 2.
Transesophageal echocardiography (aortic valve, long-axis view, 135 degrees), showing aortic valve vegetation.
Three days later, the patient suffered from a second inferior STEMI, with complete heart block. She underwent another coronary angiography that showed a large new embolus occluding the proximal segment of the right coronary artery (Video 4
 , view video online). Aspiration thrombectomy was performed again, and a temporary transvenous pacemaker was inserted. A follow-up TEE revealed that the aortic mass had shrunk from 21 × 9 mm to 16 × 9 mm, consistent with a second embolic event. A few hours later, right lower-limb ischemia was also noted. Subsequent investigations revealed thrombosis of the right femoral artery, possibly due to in situ thrombus from a hypercoagulable state and the presence of a foreign body (an Angio-Seal, Terumo Medical Corporation, Somerset, NJ) vascular closure device was deployed during the first coronary procedure) or an embolus, given the close temporal proximity to the second right coronary artery embolic event. She underwent urgent vascular surgery, with thrombectomy and removal of the Angio-Seal. Pathology of the specimen revealed a white clot composed of fibrin, and no neoplastic cells. Following surgery, the patient remained intubated due to her unstable condition.
, view video online). Aspiration thrombectomy was performed again, and a temporary transvenous pacemaker was inserted. A follow-up TEE revealed that the aortic mass had shrunk from 21 × 9 mm to 16 × 9 mm, consistent with a second embolic event. A few hours later, right lower-limb ischemia was also noted. Subsequent investigations revealed thrombosis of the right femoral artery, possibly due to in situ thrombus from a hypercoagulable state and the presence of a foreign body (an Angio-Seal, Terumo Medical Corporation, Somerset, NJ) vascular closure device was deployed during the first coronary procedure) or an embolus, given the close temporal proximity to the second right coronary artery embolic event. She underwent urgent vascular surgery, with thrombectomy and removal of the Angio-Seal. Pathology of the specimen revealed a white clot composed of fibrin, and no neoplastic cells. Following surgery, the patient remained intubated due to her unstable condition.
On day 4, she was transferred to the cardiac intensive care unit of our institution. Cardiac surgery was immediately consulted and deemed the patient to be too unstable for surgery. Indeed, the patient had developed cardiogenic shock due to right-sided heart failure following her second inferior STEMI. She required vasopressors and inotropes for hemodynamic support (norepinephrine, epinephrine, and milrinone). The patient initially was treated with intravenous heparin with a target partial thromboplastin time (PTT) between 49 and 70 seconds, per the hospital’s protocol, but the target was then modified to an anti-Xa level ranging between 0.7 and 1.0 U/mL. The complete heart block persisted, and a permanent dual-chamber pacemaker was therefore implanted on day 8. The patient slowly recovered from her heart failure and was weaned off vasopressors on day 10. A follow-up TEE showed a left ventricular ejection fraction of 60%, without regional wall-motion abnormalities, and with moderate right ventricle dilatation and hypokinesia. The mass on the aortic valve was then measured at 9 × 11 mm. No perivalvular abscess was present. The patient was successfully extubated on day 12. Throughout her hospital stay, she developed severe renal failure, attributed to acute tubular injury requiring continuous veno-venous hemodiafiltration from day 4 to day 16. An abdominal CT scan did not demonstrate renal embolism. On day 16, continuous veno-venous hemodiafiltration was replaced by intermittent hemodialysis. In the following weeks, a thrombus was found near the dialysis catheter on the right internal jugular vein. She also developed a catheter-related thrombosis in a superficial vein of the left arm. Both of these thrombotic events occurred while she was still under therapeutic anticoagulation. On day 18, warfarin was started with bridging heparin.
The patient was transferred to the internal medicine ward and was investigated thoroughly for an underlying cause. SARS-CoV-2 polymerase chain reaction analyses from a nasopharyngeal swab on day 11 and day 15 were still positive with a residual low amount of viral RNA. The patient did not receive any specific antiviral therapy for COVID-19. All blood cultures were negative, including Myco/F Lytic cultures for mycobacterium. Additional serologies for Coxiella burnetii, Bartonella henselae, hepatitis, and human immunodeficiency virus (HIV) were negative. Workup for rheumatologic and inflammatory diseases showed they were noncontributory. C3 was within the normal range, but C4 was low, at < 0.08 g/L (0.13-0.67). An antinuclear antibody test (ANA) was positive, at a titer of 1/160 (diffuse and granular staining patterns). However, an extractable nuclear antigen (ENA) screen, and tests for anti-double stranded (ds)DNA, peripheral antineutrophil cytoplasmic antibodies (p-ANCA), cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA), rheumatoid factor, cryoglobulins, and human leukocyte antigen B51 (HLA-B51) were negative. The diluted Russell viper venom time to test for lupus anticoagulant was positive (+1.40), but this result was interpreted as a false positive, because it was measured while the patient was being administered intravenous heparin. The partial thromboplastin time-lupus anticoagulant (PTTLA) test under Hepzyme (Siemens, Marburg, Germany) was normal. The lupus anticoagulant IgM and IgG tests were negative. The rest of the thrombophilia workup was negative (anticardiolipin, anti-B2-glycoprotein, JAK2, CALR, factor V, prothrombin, antithrombin, and anti-heparin antibodies). A search for an underlying malignancy revealed no neoplasia. She underwent a CT scan of her chest, abdomen, and pelvis, a mammogram, a breast ultrasound, a pelvic ultrasound, and finally a full-body positron emission tomography (PET) scan.
The patient was discharged home on day 32. She recovered adequate renal function, and intermittent hemodialysis was no longer required. The mass on the aortic valve was measured at 7 × 4 mm on a follow-up TEE 3 months after discharge and had completely disappeared at the 12-month follow-up TEE (Videos 5 and 6
 , view videos online).
, view videos online).
Almost 18 months after her initial hospitalization, the patient complained of abdominal pain. An abdominal CT scan using contrast showed a polylobulated mass measuring 3.9 × 2.8 × 3.8 cm in the jejunum, suggesting a neuroendocrine/carcinoid tumour or a gastrointestinal stromal tumour (GIST). This lesion had not been seen on her initial CT scan, which was done without contrast, due to her kidney failure. The 24-hour urine collection tests for 5-hydroxyindoleacetic acid (5-HIAA), cathecholamines, and metanephrines were normal. A dotatate PET/CT scan showed mild metabolic activity of the jejunal lesion that was absent on her first PET scan. The patient is scheduled for an upcoming small-bowel resection surgery.
Discussion
COVID-19 leads to a hypercoagulable state that predisposes patients to thromboembolism. Thrombotic events occur in the arterial circulation in 1%-5% of cases and may cause stroke, acute coronary syndrome, or limb ischemia. The pathophysiology of COVID-19-associated coagulopathy is complex and implies different pathways that occur in parallel, leading to thrombus formation.2
Regarding this case report, a reasonable question is whether the malignancy was already present during her thromboembolic events, but was missed on the initial scans, or rather it developed afterwards. However, cases of marantic endocarditis usually are found in patients with already advanced malignancies. In the only 2 case reports of NBTE with COVID-19 and malignancy, 1 had a recent diagnosis of stage IV gastric cancer,3 and the other had stage IV prostate cancer found during the etiology workup of his NBTE.5 In our case, the first PET scan did not detect any suspect lesion. Hence, whether the patient’s hypercoagulable state was due solely to COVID-19-associated coagulopathy, or to an insidious neoplasia, or to a combination of both is difficult to determine.
According to the American College of Chest Physicians (CHEST), treatment with a therapeutic dose of intravenous unfractionated heparin or low-molecular-weight heparin is recommended for patients with NBTE with systemic or pulmonary emboli (grade 2c). No trials have compared heparin and warfarin specifically in patients with NBTE, but some studies have shown benefits with warfarin. The use of direct oral anticoagulants (DOACs) has not been studied to support their routine use in NBTE.1,7 In general, in COVID-19 patients, unfractionated heparin and low-molecular-weight heparin are also preferred over DOACs, because of the drug interaction between DOACs and many medications used for COVID-19 treatment, such as dexamethasone and antivirals.2 As no specific guidelines have been developed on anticoagulation for marantic endocarditis in COVID-19 patients, a decision was made by the hematology team, based on the specifics of her case, for our patient to use lifelong anticoagulation with warfarin. Warfarin was chosen over heparin because the patient initially had an abnormal diluted Russell viper venom time (for lupus anticoagulant), although this result was considered a false positive. With the discovery of her intestinal tumour, and given her upcoming surgery, the use and duration of warfarin will need to be reassessed during medical follow-ups.
Conclusion
Thromboembolic events are seen frequently in patients with COVID-19. We describe here a patient with COVID-19 who presented with marantic endocarditis and recurrent thromboembolic events. Extensive investigations ruled out other causes for the hypercoagulable state, such as malignancy and inflammatory diseases. However, a neoplasia was diagnosed 18 months later. Given this case, we suggest that marantic endocarditis can be an atypical presentation of COVID-19 and possibly of delayed-onset malignancy.
Novel Teaching Points.
-
•
Marantic endocarditis carries a high risk of systemic thromboembolism, including coronary embolism.
-
•
COVID-19 leads to a hypercoagulable state that can present as marantic endocarditis, even in the absence of both an advanced malignancy and inflammatory disease.
-
•
Marantic endocarditis associated with COVID-19 remains a diagnosis of exclusion. A workup to find a cause for the hypercoagulable state is warranted.
-
•
A thorough medical follow-up in patients with NBTE is crucial, as an insidious-onset or delayed-onset malignancy can be discovered at a later point.
Acknowledgments
Ethics Statement
The authors declared that this case report has adhered to the relevant local ethical guidelines.
Patient Consent
The authors confirm that patient consent is not applicable to this article. This is a retrospective case report using de-identified data; therefore the IRB did not require consent from the patient.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 665 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.12.017.
Supplementary Material
First RCA angiogram, LAO 30 degrees.
Transesophageal echocardiography initial diagnosis, Aortic valve long axis, 135 degrees.
Transesophageal echocardiography initial diagnosis, Aortic valve short axis, 70 degrees
Second RCA angiogram, LAO 30 degrees.
Transesophageal echocardiography 3-month follow-up, Aortic valve long axis, 130 degrees.
Transesophageal echocardiography 1-year follow-up, Aortic valve long axis, 135 degrees.
References
- 1.Liu J., Frishman W.H. Nonbacterial thrombotic endocarditis: pathogenesis, diagnosis, and management. Cardiol Rev. 2016;24:244–247. doi: 10.1097/CRD.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health COVID-19 treatment guidelines: antithrombotic therapy in patients with COVID-19. https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/ Available at:
- 3.Binet Q., Goffinet C., Etogo-Asse F.E., Shaza L. Nonbacterial thrombotic endocarditis in a patient with gastric cancer and SARS-CoV-2 infection. Clin J Gastroenterol. 2021;14:1031–1035. doi: 10.1007/s12328-021-01412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan K.H., Joseph O., Ahmed E., et al. Marantic endocarditis associated with COVID-19: a rare case report of a potentially deadly disease. Eur J Case Rep Intern Med. 2021;8 doi: 10.12890/2021_002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Herck J., Thoen H., Delens C., Voet J. Multi-territory stroke preceded by pulmonary embolism with asymptomatic coronavirus disease 2019: a case report. Eur Heart J Case Rep. 2021;5 doi: 10.1093/ehjcr/ytab471. ytab471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad A., Golemi L., Bedi R., et al. Nonbacterial thrombotic endocarditis in a Covid-19 patient [abstract] JACC. 2023;81(suppl A):2997. [Google Scholar]
- 7.Whitlock R.P., Sun J.C., Fremes S.E., Rubens F.D., Teoh K.H. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e576S–e600S. doi: 10.1378/chest.11-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
First RCA angiogram, LAO 30 degrees.
Transesophageal echocardiography initial diagnosis, Aortic valve long axis, 135 degrees.
Transesophageal echocardiography initial diagnosis, Aortic valve short axis, 70 degrees
Second RCA angiogram, LAO 30 degrees.
Transesophageal echocardiography 3-month follow-up, Aortic valve long axis, 130 degrees.
Transesophageal echocardiography 1-year follow-up, Aortic valve long axis, 135 degrees.