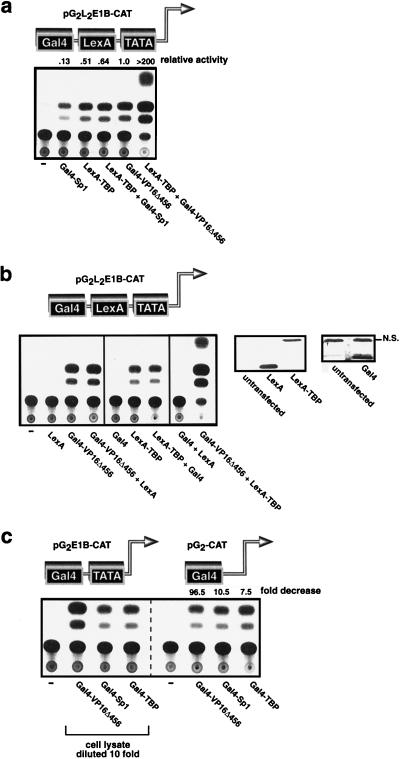
FIG. 2.
Sp1 recruits TBP, and VP16Δ456 targets a step that is downstream of TBP recruitment to activate transcription. (a) Gal4-Sp1 is inert if TBP is tethered to the promoter by LexA, but Gal4-VP16Δ456 cooperates with LexA-TBP to activate transcription synergistically. (b) Synergistic activation induced by the combination of Gal4-VP16Δ456 and LexA-TBP is dependent upon the VP16Δ456 and TBP moieties in these chimeric proteins. This is evidenced by the fact that the Gal4 and LexA DNA-binding domains are inactive in this assay (left panel) even though the Gal4 and LexA DNA-binding domains are readily expressed in transfection assays when assessed by immunoblotting (right panels). NS, nonspecific bond. (c) Gal4-Sp1 and Gal4-TBP are less dependent upon the TATA box than Gal4-VP16Δ456 for their transactivation function. “fold decrease” represents the ratio of the activity of each activator in the presence to that in the absence of the TATA box. The cell lysates for the assays depicted in the left panel were diluted 10-fold to facilitate the comparison between the two reporter constructs.